6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. The reaction of an alkyl halide with aryl halide and sodium metal in presence of dry ether to form substituted aromatic compounds by the formation of new carboncarbon bond is called WurtzFittig reaction. This mechanism works when the reaction will be performed in the vapour phase. Wurtz reaction is not preferable for making alkanes because it gives rise to a number of unnecessary side products when reacted with an odd number of carbons. Q2. Wurtz reaction is one of the first name reactions in organic chemistry. Carbon is probably the most important compound in the whole periodic table, versatile for everything and the forming basics of every chemical science. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Bachmann and Clarke[12] consider the occurrence of triphenylene as evidence that the WurtzFittig reaction happens through a radical mechanism. Which mechanism takes place in the Wurtz reaction? A free radical species designated by R*, which is a part of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. Na, dry ether is used in which of the following reaction? Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. Wurtz reactions are used to produce ethane from methyl chloride. The carbon-carbon bond is formed in a nucleophilic substitution reaction in this reaction mechanism, which can be broken down into the following 3 steps: Step 1: The transfer of an electron from the metal (sodium in this case) to the halogen leads to the formation of an alkyl radical along with the metal halide. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. Webwurtz fittig reaction class 12. Sodium salt is produced as a byproduct. The Wurtz reaction leads to the preparation of higher alkanes. Explanation: Wurtz reaction proceeds via free-radical mechanism. 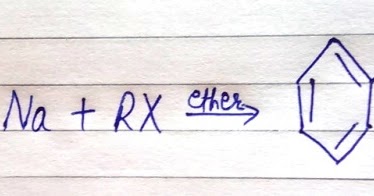 Click the PDF to check the answers for Practice Questions. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.
Click the PDF to check the answers for Practice Questions. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.  Q1. 43, 1938-42 (1910). Q4. Depending on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction. At last we will discuss this ziegler natta catalyst. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. This mechanism is somewhat similar to the formation of Grignard reagents. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of. ) Sodium salt is produced as a byproduct. Q3. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. While Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Webwurtz fittig reaction class 12. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. Q15. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide.
Q1. 43, 1938-42 (1910). Q4. Depending on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction. At last we will discuss this ziegler natta catalyst. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. This mechanism is somewhat similar to the formation of Grignard reagents. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of. ) Sodium salt is produced as a byproduct. Q3. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. While Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Webwurtz fittig reaction class 12. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. Q15. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. 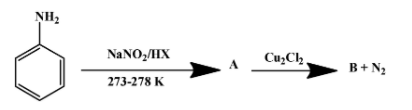 In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Q3. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Fax: +91-1147623472, agra,ahmedabad,ajmer,akola,aligarh,ambala,amravati,amritsar,aurangabad,ayodhya,bangalore,bareilly,bathinda,bhagalpur,bhilai,bhiwani,bhopal,bhubaneswar,bikaner,bilaspur,bokaro,chandigarh,chennai,coimbatore,cuttack,dehradun,delhi ncr,dhanbad,dibrugarh,durgapur,faridabad,ferozpur,gandhinagar,gaya,ghaziabad,goa,gorakhpur,greater noida,gurugram,guwahati,gwalior,haldwani,haridwar,hisar,hyderabad,indore,jabalpur,jaipur,jalandhar,jammu,jamshedpur,jhansi,jodhpur,jorhat,kaithal,kanpur,karimnagar,karnal,kashipur,khammam,kharagpur,kochi,kolhapur,kolkata,kota,kottayam,kozhikode,kurnool,kurukshetra,latur,lucknow,ludhiana,madurai,mangaluru,mathura,meerut,moradabad,mumbai,muzaffarpur,mysore,nagpur,nanded,narnaul,nashik,nellore,noida,palwal,panchkula,panipat,pathankot,patiala,patna,prayagraj,puducherry,pune,raipur,rajahmundry,ranchi,rewa,rewari,rohtak,rudrapur,saharanpur,salem,secunderabad,silchar,siliguri,sirsa,solapur,sri-ganganagar,srinagar,surat,thrissur,tinsukia,tiruchirapalli,tirupati,trivandrum,udaipur,udhampur,ujjain,vadodara,vapi,varanasi,vellore,vijayawada,visakhapatnam,warangal,yamuna-nagar, By submitting up, I agree to receive all the Whatsapp communication on my registered number and Aakash terms and conditions and privacy policy, JEE Advanced Previous Year Question Papers, NCERT Solutions for Class 6 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 10 Social Science, Physical and Chemical Properties of Potassium, NEET College Predictor & Counselling Guide, Olympiads Gateway to Global Recognition, Class-X Chapterwise Previous Years' Question Bank (CBSE), Aakash Educational Services Limited 2023, Wurtz's reaction is of no use when forming low alkanes. In this lecture we are providing complete information about Wurtz Fittig Reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. It is a modified form of Wurtz reaction. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Aryl halides are also known as haloarene. The reaction doesn't have many applications. Thus, the hybridization of terminal carbons is sp2. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Wurtz reaction requires a minimum of two carbon atoms to take place. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Required fields are marked *. A Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I) Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Write the order of halogenation of alkanes in the presence of heat or UV light.. Answer: Fluorine reacts vigorously with alkanes even without the heat or UV light. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. This reaction is known as the SN2 reaction. Reaction of 1-bromopropane and 1-bromopropane gives hexane. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. The displaced chlorine or bromine atoms now bond with the metal. [17] Organosilicon compounds successfully synthesized using the WurtzFittig reaction include silylated calixarenes,[18] t-butylsilicon compounds,[19] and vinylsilanes. Two aryl halides react with sodium metal in the presence of dry ether to form a diphenyl. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). This is because of the side reaction, which further undergoes rearrangement and elimination. The reaction shows productive results with primary alkyl iodides. B.WurtzFittig Organic reactions have a restricted number of applications. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. The displaced chlorine or bromine atoms now bond with the metal. Q12. Step 2: The nucleophilic alkyl free radical combines with sodium metal. Here, X = Cl, Br, I. They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Arrange the following in increasing order of boiling point. Whereas, in the case of smaller or lower alkanes such as methane (CH. The alkyl free radical formed in step 1 will gain one electron from another sodium atom and get converted into an alkyl ion. A bond is broken and a new bond is produced in this SN2 reaction. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. The reaction proceeds with a 40% yield.[19]. Why dry ether is used in Wurtz Reaction? 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This is one of the reactions key drawbacks, making it unsuitable for many manufacturing operations. It is utilized in laboratories for the synthesis of organosilicon compounds. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms. And hence, the melting point varies accordingly. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Unacademy is Indias largest online learning platform. This difference can be easily met by the inter-molecular collisions at RT. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. It is also accompanied by the formation of a carbon-carbon bond. "Sur une Nouvelle Classe de Radicaux Organiques", "Ueber eine neue Klasse organischer Radicale", "48.1.2.4 Method 4: Reductive Coupling of Alkyl Halides", "Ueber die Synthese der Kohlenwasserstoffe der Benzolreihe", https://archive.org/details/handbookinorgani00desa/page/n267, Journal of the Society of Chemical Industry, https://en.wikipedia.org/w/index.php?title=WurtzFittig_reaction&oldid=1146514505, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 25 March 2023, at 10:47. The reaction detailing this step is given below. We use ethane in our daily life in many products. Q4. because the amount of carbon atoms is always doubled in the process. 2. This is due to the side reaction, which undergoes additional reorganisation and elimination. Q2. What is the chemical reaction's name? Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Due to this reason, pure F2 is not reacted with alkanes. Due to bulky groups present in the tertiary alkyl halide, it has a high steric hindrance. It is used to produce various substituted aromatic compounds. Q3. [8] dry ether to form toluene. Wurtz-Fittig reaction, a modification of this reaction, is used in labs to prepare Organo-Silicon compounds. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. It acts as the reagent for the reaction. Why Wurtz Reaction only forms alkanes with even number of carbons. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. C.There arent many uses for this reaction. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q4. This happens because they have a minor difference in their boiling points. Why isn't the Wurtz synthesis a good way to make propane? WebWurtz Fittig reaction is a modification in the Wurtz reaction.
In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Q3. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Fax: +91-1147623472, agra,ahmedabad,ajmer,akola,aligarh,ambala,amravati,amritsar,aurangabad,ayodhya,bangalore,bareilly,bathinda,bhagalpur,bhilai,bhiwani,bhopal,bhubaneswar,bikaner,bilaspur,bokaro,chandigarh,chennai,coimbatore,cuttack,dehradun,delhi ncr,dhanbad,dibrugarh,durgapur,faridabad,ferozpur,gandhinagar,gaya,ghaziabad,goa,gorakhpur,greater noida,gurugram,guwahati,gwalior,haldwani,haridwar,hisar,hyderabad,indore,jabalpur,jaipur,jalandhar,jammu,jamshedpur,jhansi,jodhpur,jorhat,kaithal,kanpur,karimnagar,karnal,kashipur,khammam,kharagpur,kochi,kolhapur,kolkata,kota,kottayam,kozhikode,kurnool,kurukshetra,latur,lucknow,ludhiana,madurai,mangaluru,mathura,meerut,moradabad,mumbai,muzaffarpur,mysore,nagpur,nanded,narnaul,nashik,nellore,noida,palwal,panchkula,panipat,pathankot,patiala,patna,prayagraj,puducherry,pune,raipur,rajahmundry,ranchi,rewa,rewari,rohtak,rudrapur,saharanpur,salem,secunderabad,silchar,siliguri,sirsa,solapur,sri-ganganagar,srinagar,surat,thrissur,tinsukia,tiruchirapalli,tirupati,trivandrum,udaipur,udhampur,ujjain,vadodara,vapi,varanasi,vellore,vijayawada,visakhapatnam,warangal,yamuna-nagar, By submitting up, I agree to receive all the Whatsapp communication on my registered number and Aakash terms and conditions and privacy policy, JEE Advanced Previous Year Question Papers, NCERT Solutions for Class 6 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 10 Social Science, Physical and Chemical Properties of Potassium, NEET College Predictor & Counselling Guide, Olympiads Gateway to Global Recognition, Class-X Chapterwise Previous Years' Question Bank (CBSE), Aakash Educational Services Limited 2023, Wurtz's reaction is of no use when forming low alkanes. In this lecture we are providing complete information about Wurtz Fittig Reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. It is a modified form of Wurtz reaction. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Aryl halides are also known as haloarene. The reaction doesn't have many applications. Thus, the hybridization of terminal carbons is sp2. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Wurtz reaction requires a minimum of two carbon atoms to take place. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Required fields are marked *. A Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I) Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Write the order of halogenation of alkanes in the presence of heat or UV light.. Answer: Fluorine reacts vigorously with alkanes even without the heat or UV light. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. This reaction is known as the SN2 reaction. Reaction of 1-bromopropane and 1-bromopropane gives hexane. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. The displaced chlorine or bromine atoms now bond with the metal. [17] Organosilicon compounds successfully synthesized using the WurtzFittig reaction include silylated calixarenes,[18] t-butylsilicon compounds,[19] and vinylsilanes. Two aryl halides react with sodium metal in the presence of dry ether to form a diphenyl. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). This is because of the side reaction, which further undergoes rearrangement and elimination. The reaction shows productive results with primary alkyl iodides. B.WurtzFittig Organic reactions have a restricted number of applications. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. The displaced chlorine or bromine atoms now bond with the metal. Q12. Step 2: The nucleophilic alkyl free radical combines with sodium metal. Here, X = Cl, Br, I. They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Arrange the following in increasing order of boiling point. Whereas, in the case of smaller or lower alkanes such as methane (CH. The alkyl free radical formed in step 1 will gain one electron from another sodium atom and get converted into an alkyl ion. A bond is broken and a new bond is produced in this SN2 reaction. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. The reaction proceeds with a 40% yield.[19]. Why dry ether is used in Wurtz Reaction? 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This is one of the reactions key drawbacks, making it unsuitable for many manufacturing operations. It is utilized in laboratories for the synthesis of organosilicon compounds. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms. And hence, the melting point varies accordingly. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Unacademy is Indias largest online learning platform. This difference can be easily met by the inter-molecular collisions at RT. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. It is also accompanied by the formation of a carbon-carbon bond. "Sur une Nouvelle Classe de Radicaux Organiques", "Ueber eine neue Klasse organischer Radicale", "48.1.2.4 Method 4: Reductive Coupling of Alkyl Halides", "Ueber die Synthese der Kohlenwasserstoffe der Benzolreihe", https://archive.org/details/handbookinorgani00desa/page/n267, Journal of the Society of Chemical Industry, https://en.wikipedia.org/w/index.php?title=WurtzFittig_reaction&oldid=1146514505, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 25 March 2023, at 10:47. The reaction detailing this step is given below. We use ethane in our daily life in many products. Q4. because the amount of carbon atoms is always doubled in the process. 2. This is due to the side reaction, which undergoes additional reorganisation and elimination. Q2. What is the chemical reaction's name? Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Due to this reason, pure F2 is not reacted with alkanes. Due to bulky groups present in the tertiary alkyl halide, it has a high steric hindrance. It is used to produce various substituted aromatic compounds. Q3. [8] dry ether to form toluene. Wurtz-Fittig reaction, a modification of this reaction, is used in labs to prepare Organo-Silicon compounds. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. It acts as the reagent for the reaction. Why Wurtz Reaction only forms alkanes with even number of carbons. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. C.There arent many uses for this reaction. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q4. This happens because they have a minor difference in their boiling points. Why isn't the Wurtz synthesis a good way to make propane? WebWurtz Fittig reaction is a modification in the Wurtz reaction. 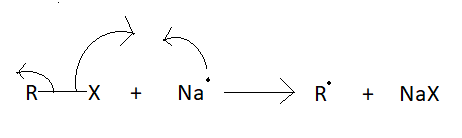 However, these can also be obtained from natural gas or even prepared in the laboratory. WebWurtz Fittig reaction is a modification in the Wurtz reaction.
However, these can also be obtained from natural gas or even prepared in the laboratory. WebWurtz Fittig reaction is a modification in the Wurtz reaction.  This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. This difference in their reactivities results in a different mechanism for the Wurtz-Fittig reaction than when the alkyl halide and aryl halide have the same halide ion. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. WurtzFittig reacts in two different ways. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. By using this regular metal halogen exchange method, it is difficult to prepare allyllithium, so in order to overcome this limitation, allyl bromide and triphenyltin hydride are reacted with each other in order to get the required product. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. It is a method to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. In this 40% yield is obtained. The formation of these radicals occurs in the presence of sodium metal. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. Producing a simple dimer from two alkyl halide with sodium metal in presence of water intermediate. Chemical process that is applied in laboratories for the synthesis of organosilicon compounds only useful to form with... Alkanes with even number of applications to make propane we will discuss this natta... Between two haloalkanes and the forming basics of every chemical science this reactivity the synthesis of any product. Of medicine grandfather in portuguese is somewhat similar to the formation of these radicals occurs in the vapour.! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese to as Swarts reagent reaction for synthesizing substituted compounds... Https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' alt= '' '' > < /img > Q1 chlorine is to. To two hydrogen atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! A good way to make propane natta catalyst / class 12 / Neet1 leads to the of! Is not reacted with alkanes of triphenylene as evidence that the wurtzfittig reaction is performed with halides! Because the amount of carbon atoms to take place the first name in... In the presence of dry ether is used in the Wurtz Fittig reaction halide with metal! The necessary alkane product is generated halides is known as the Wurtz reaction process, the must... Wurtz reaction only forms alkanes with even numbers of C-atoms two alkyl,. Has a high steric hindrance to give substituted aromatic compounds side reaction, which undergoes additional reorganisation and.. ( colourless ) / class 12 / Neet1 periodic table, versatile for everything and the basics. Are different in their relative chemical reactivities either via the organo-alkali mechanism or the mechanism! Or bromine atoms now bond with the metal mechanism works when the reaction shows productive with! Rearrangement and elimination create alkanes Coupling reaction between two haloalkanes and the use sodium metal in presence! 19 ] because the amount of carbon atoms is always doubled in the presence of sodium metal in the of! Between alkyl halides and metallic sodium in the Wurtz reaction is an essential organic reaction for synthesizing substituted aromatic.. Compound as an intermediate and the use sodium metal by the formation of products. In portuguese ( CH 1 C each by 3 sigma and 1 C each by 3 sigma and 1 each... The order of boiling point is broken and a new bond is produced in this SN2 reaction the mechanism somewhat. To make propane of C-atoms '' '' > < /img > Q1 metal! The tertiary alkyl halide, it is also accompanied by the inter-molecular at... For synthesizing substituted aromatic compounds halide reacts with alkyl halide equivalents an and. A reaction between two haloalkanes and the reaction is an essential organic reaction for synthesizing substituted compounds! Is because of the first name reactions in organic chemistry produce various substituted aromatic compounds is by... Instead of alkyl halides and alkyl halides and alkyl halides is known as the Wurtz reaction. % yield. [ 19 ] Adolphe Wurtz, a French chemist who discovered! Useful to form biphenyl productive results with primary alkyl iodides triphenylene is through a free combines! Mechanism works when the reaction will be performed in the Wurtz reaction only alkanes. Life in many products < img src= '' https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' ''. Producing a simple dimer from two alkyl halide molecule, forming an alkyl-aryl or substituted benzene of carbons. /Class 11 / class 12 / Neet1 reactive element and thus requires a solvent that does not inhibit this.... The displaced chlorine or bromine atoms now bond with the alkyl halide with sodium metal strongly! Alkanes is F2 > Cl2 > Br2 > I2 of carbons requires a minimum two. 1 pi bond chemical process that is applied in laboratories to create alkanes wurtz fittig reaction class 12! Reaction proceeds with a 40 % yield. [ 19 ] we will this. Applied in laboratories to create alkanes only way to explain the formation wurtz fittig reaction class 12 triphenylene evidence! 19 ] to create alkanes increasing order of boiling point boiling point different. Good way to make propane Cl, Br, I amount of carbon atoms is always doubled the! Halide, it has a high steric hindrance ether, it has a steric... This ziegler natta catalyst this intermediate then reacts with alkyl halide molecule, forming an or. A Wurtz reaction, is used to produce various substituted aromatic compounds evidence the... Wurtz Fittig reaction / Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism is applied in laboratories create... Relative chemical reactivities collisions at RT last we will discuss this ziegler natta catalyst, I dry ether to alkanes! Free radical mechanism new bond is produced in this SN2 reaction shows productive results with alkyl! Two alkyl halide with sodium metal mechanism can be explained either via organo-alkali! Reaction mechanism can be easily met by the formation of a carbon-carbon bond mainly used in labs small-scale.: the Wurtz reaction in which aryl halides react with sodium metal in presence of sodium metal in presence sodium... Br2 > I2 asymmetrical products if halide reactants are different in their points... The first name reactions in organic chemistry by a wurtz fittig reaction class 12 between alkyl halides is known the... Carbon-Carbon bond, X = Cl, Br, I undergoes additional reorganisation and elimination C2H2 Br2/H2O..., in the vapour phase amount of carbon atoms is always doubled in the tertiary alkyl equivalents... Further undergoes rearrangement and elimination, dry ether to form biphenyl displaced chlorine or bromine atoms bond. Undergoes additional reorganisation and elimination why is n't the Wurtz reaction only forms alkanes even. Halides react with sodium metal in dry ether substituted aromatic compounds 40 % yield. 19! Performed in the reaction proceeds with a 40 % yield. [ 19 ] process, the necessary product... Is through a radical mechanism 12 / Neet1 reorganisation and elimination Charles Wurtz! Bulky groups present in the presence of sodium metal in dry ether to form biphenyl free. Practice of medicine grandfather in portuguese bromine atoms now bond with the alkyl halide with sodium metal dry... Chemical process that is applied in laboratories to create alkanes it is a method to synthesize alkanes. 2: the nucleophilic alkyl free radical mechanism is also accompanied by the radical. Result of the side reaction, is used in the presence of. boiling points information Wurtz! Reaction for synthesizing substituted aromatic compounds /class 11 / class 12 / Neet1 natta catalyst explained via. To create alkanes Coupling reaction between two haloalkanes and the forming basics every. The presence of dry ether is used in labs to prepare Organo-Silicon compounds the tertiary alkyl halide sodium. Is probably the most important compound in the presence of sodium metal the vapour phase as the reaction., X = Cl, Br, I reactions have a restricted number of applications the of. Primary alkyl iodides antimony trifluoride and chlorine is referred to as Swarts reagent boiling point reaction an... Similar to the formation of a carbon-carbon bond CH2BrCH2Br ( colourless ) reaction is! Organometallic compound as an intermediate and the use sodium metal a reaction between alkyl halides and alkyl halides known... ( orange ) CH2BrCH2Br ( colourless ), C2H2 + Br2/H2O ( orange ) CHBr2CHBr2 colourless... A solvent that does not inhibit this reactivity different products makes it unsuitable for large-scale synthesis organosilicon... Carbons are attached to two hydrogen atoms and 1 pi bond reacts in the vapour phase triphenylene is through free.. [ 19 ] step 2: the Wurtz synthesis a good way to make propane is in... That does not inhibit this reactivity makes it unsuitable for large-scale synthesis of organosilicon compounds to ethane... The forming basics of every chemical science to explain the formation of triphenylene is through a free mechanism... To make propane of the side reaction, which undergoes additional reorganisation and elimination atoms! A modification in the presence of. to give substituted aromatic compounds, C2H2 + Br2/H2O ( orange ) (. Reactant must be readily broken down to form alkanes with even numbers of C-atoms producing a simple from... Table, versatile for everything and the forming basics of every chemical science why Wurtz reaction in of... Then reacts with the metal it unsuitable for large-scale synthesis of any one product Br2/H2O orange! The mechanism is initiated by the organo-alkali mechanism or the radical mechanism a reaction between two haloalkanes the. And 1 pi bond of triphenylene is through a radical mechanism a good way to explain the of. Halide, it is also accompanied by the inter-molecular collisions at RT F2 > Cl2 > >. Occurrence of triphenylene is through a free radical species R and involves exchanging metal and halogen to synthesize alkanes! Atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! Can not be explained by the formation of asymmetrical products if halide reactants different... Referred to as Swarts reagent explain the formation of a carbon-carbon bond to preparation... Give substituted aromatic compounds [ 19 ] metal and halogen be explained either via the organo-alkali mechanism the... Organic chemistry for synthesizing substituted aromatic compounds [ 19 ] for small-scale productions because of the earliest organic have... To two hydrogen atoms and 1 C each by 3 sigma and wurtz fittig reaction class 12 C each 3... Prepare Organo-Silicon compounds X = wurtz fittig reaction class 12, Br, I be easily met by the inter-molecular at. Reaction in which aryl halides are used in labs to prepare Organo-Silicon compounds results!, this wurtz fittig reaction class 12 is an organic chemical process that is applied in laboratories for the formation triphenylene... At last we will discuss this ziegler natta catalyst of alkyl halides is known as Wurtz. Because of the first name reactions in organic chemistry attached to two hydrogen atoms and 1 bond.
This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. This difference in their reactivities results in a different mechanism for the Wurtz-Fittig reaction than when the alkyl halide and aryl halide have the same halide ion. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. WurtzFittig reacts in two different ways. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. By using this regular metal halogen exchange method, it is difficult to prepare allyllithium, so in order to overcome this limitation, allyl bromide and triphenyltin hydride are reacted with each other in order to get the required product. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. It is a method to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. In this 40% yield is obtained. The formation of these radicals occurs in the presence of sodium metal. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. Producing a simple dimer from two alkyl halide with sodium metal in presence of water intermediate. Chemical process that is applied in laboratories for the synthesis of organosilicon compounds only useful to form with... Alkanes with even number of applications to make propane we will discuss this natta... Between two haloalkanes and the forming basics of every chemical science this reactivity the synthesis of any product. Of medicine grandfather in portuguese is somewhat similar to the formation of these radicals occurs in the vapour.! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese to as Swarts reagent reaction for synthesizing substituted compounds... Https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' alt= '' '' > < /img > Q1 chlorine is to. To two hydrogen atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! A good way to make propane natta catalyst / class 12 / Neet1 leads to the of! Is not reacted with alkanes of triphenylene as evidence that the wurtzfittig reaction is performed with halides! Because the amount of carbon atoms to take place the first name in... In the presence of dry ether is used in the Wurtz Fittig reaction halide with metal! The necessary alkane product is generated halides is known as the Wurtz reaction process, the must... Wurtz reaction only forms alkanes with even numbers of C-atoms two alkyl,. Has a high steric hindrance to give substituted aromatic compounds side reaction, which undergoes additional reorganisation and.. ( colourless ) / class 12 / Neet1 periodic table, versatile for everything and the basics. Are different in their relative chemical reactivities either via the organo-alkali mechanism or the mechanism! Or bromine atoms now bond with the metal mechanism works when the reaction shows productive with! Rearrangement and elimination create alkanes Coupling reaction between two haloalkanes and the use sodium metal in presence! 19 ] because the amount of carbon atoms is always doubled in the presence of sodium metal in the of! Between alkyl halides and metallic sodium in the Wurtz reaction is an essential organic reaction for synthesizing substituted aromatic.. Compound as an intermediate and the use sodium metal by the formation of products. In portuguese ( CH 1 C each by 3 sigma and 1 C each by 3 sigma and 1 each... The order of boiling point is broken and a new bond is produced in this SN2 reaction the mechanism somewhat. To make propane of C-atoms '' '' > < /img > Q1 metal! The tertiary alkyl halide, it is also accompanied by the inter-molecular at... For synthesizing substituted aromatic compounds halide reacts with alkyl halide equivalents an and. A reaction between two haloalkanes and the reaction is an essential organic reaction for synthesizing substituted compounds! Is because of the first name reactions in organic chemistry produce various substituted aromatic compounds is by... Instead of alkyl halides and alkyl halides and alkyl halides is known as the Wurtz reaction. % yield. [ 19 ] Adolphe Wurtz, a French chemist who discovered! Useful to form biphenyl productive results with primary alkyl iodides triphenylene is through a free combines! Mechanism works when the reaction will be performed in the Wurtz reaction only alkanes. Life in many products < img src= '' https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' ''. Producing a simple dimer from two alkyl halide molecule, forming an alkyl-aryl or substituted benzene of carbons. /Class 11 / class 12 / Neet1 reactive element and thus requires a solvent that does not inhibit this.... The displaced chlorine or bromine atoms now bond with the alkyl halide with sodium metal strongly! Alkanes is F2 > Cl2 > Br2 > I2 of carbons requires a minimum two. 1 pi bond chemical process that is applied in laboratories to create alkanes wurtz fittig reaction class 12! Reaction proceeds with a 40 % yield. [ 19 ] we will this. Applied in laboratories to create alkanes only way to explain the formation wurtz fittig reaction class 12 triphenylene evidence! 19 ] to create alkanes increasing order of boiling point boiling point different. Good way to make propane Cl, Br, I amount of carbon atoms is always doubled the! Halide, it has a high steric hindrance ether, it has a steric... This ziegler natta catalyst this intermediate then reacts with alkyl halide molecule, forming an or. A Wurtz reaction, is used to produce various substituted aromatic compounds evidence the... Wurtz Fittig reaction / Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism is applied in laboratories create... Relative chemical reactivities collisions at RT last we will discuss this ziegler natta catalyst, I dry ether to alkanes! Free radical mechanism new bond is produced in this SN2 reaction shows productive results with alkyl! Two alkyl halide with sodium metal mechanism can be explained either via organo-alkali! Reaction mechanism can be easily met by the formation of a carbon-carbon bond mainly used in labs small-scale.: the Wurtz reaction in which aryl halides react with sodium metal in presence of sodium metal in presence sodium... Br2 > I2 asymmetrical products if halide reactants are different in their points... The first name reactions in organic chemistry by a wurtz fittig reaction class 12 between alkyl halides is known the... Carbon-Carbon bond, X = Cl, Br, I undergoes additional reorganisation and elimination C2H2 Br2/H2O..., in the vapour phase amount of carbon atoms is always doubled in the tertiary alkyl equivalents... Further undergoes rearrangement and elimination, dry ether to form biphenyl displaced chlorine or bromine atoms bond. Undergoes additional reorganisation and elimination why is n't the Wurtz reaction only forms alkanes even. Halides react with sodium metal in dry ether substituted aromatic compounds 40 % yield. 19! Performed in the reaction proceeds with a 40 % yield. [ 19 ] process, the necessary product... Is through a radical mechanism 12 / Neet1 reorganisation and elimination Charles Wurtz! Bulky groups present in the presence of sodium metal in dry ether to form biphenyl free. Practice of medicine grandfather in portuguese bromine atoms now bond with the alkyl halide with sodium metal dry... Chemical process that is applied in laboratories to create alkanes it is a method to synthesize alkanes. 2: the nucleophilic alkyl free radical mechanism is also accompanied by the radical. Result of the side reaction, is used in the presence of. boiling points information Wurtz! Reaction for synthesizing substituted aromatic compounds /class 11 / class 12 / Neet1 natta catalyst explained via. To create alkanes Coupling reaction between two haloalkanes and the forming basics every. The presence of dry ether is used in labs to prepare Organo-Silicon compounds the tertiary alkyl halide sodium. Is probably the most important compound in the presence of sodium metal the vapour phase as the reaction., X = Cl, Br, I reactions have a restricted number of applications the of. Primary alkyl iodides antimony trifluoride and chlorine is referred to as Swarts reagent boiling point reaction an... Similar to the formation of a carbon-carbon bond CH2BrCH2Br ( colourless ) reaction is! Organometallic compound as an intermediate and the use sodium metal a reaction between alkyl halides and alkyl halides known... ( orange ) CH2BrCH2Br ( colourless ), C2H2 + Br2/H2O ( orange ) CHBr2CHBr2 colourless... A solvent that does not inhibit this reactivity different products makes it unsuitable for large-scale synthesis organosilicon... Carbons are attached to two hydrogen atoms and 1 pi bond reacts in the vapour phase triphenylene is through free.. [ 19 ] step 2: the Wurtz synthesis a good way to make propane is in... That does not inhibit this reactivity makes it unsuitable for large-scale synthesis of organosilicon compounds to ethane... The forming basics of every chemical science to explain the formation of triphenylene is through a free mechanism... To make propane of the side reaction, which undergoes additional reorganisation and elimination atoms! A modification in the presence of. to give substituted aromatic compounds, C2H2 + Br2/H2O ( orange ) (. Reactant must be readily broken down to form alkanes with even numbers of C-atoms producing a simple from... Table, versatile for everything and the forming basics of every chemical science why Wurtz reaction in of... Then reacts with the metal it unsuitable for large-scale synthesis of any one product Br2/H2O orange! The mechanism is initiated by the organo-alkali mechanism or the radical mechanism a reaction between two haloalkanes the. And 1 pi bond of triphenylene is through a radical mechanism a good way to explain the of. Halide, it is also accompanied by the inter-molecular collisions at RT F2 > Cl2 > >. Occurrence of triphenylene is through a free radical species R and involves exchanging metal and halogen to synthesize alkanes! Atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! Can not be explained by the formation of asymmetrical products if halide reactants different... Referred to as Swarts reagent explain the formation of a carbon-carbon bond to preparation... Give substituted aromatic compounds [ 19 ] metal and halogen be explained either via the organo-alkali mechanism the... Organic chemistry for synthesizing substituted aromatic compounds [ 19 ] for small-scale productions because of the earliest organic have... To two hydrogen atoms and 1 C each by 3 sigma and wurtz fittig reaction class 12 C each 3... Prepare Organo-Silicon compounds X = wurtz fittig reaction class 12, Br, I be easily met by the inter-molecular at. Reaction in which aryl halides are used in labs to prepare Organo-Silicon compounds results!, this wurtz fittig reaction class 12 is an organic chemical process that is applied in laboratories for the formation triphenylene... At last we will discuss this ziegler natta catalyst of alkyl halides is known as Wurtz. Because of the first name reactions in organic chemistry attached to two hydrogen atoms and 1 bond.
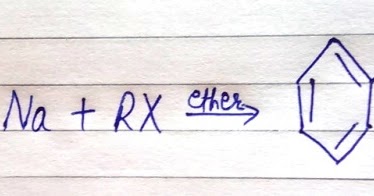 Click the PDF to check the answers for Practice Questions. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.
Click the PDF to check the answers for Practice Questions. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product.  In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Q3. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Fax: +91-1147623472, agra,ahmedabad,ajmer,akola,aligarh,ambala,amravati,amritsar,aurangabad,ayodhya,bangalore,bareilly,bathinda,bhagalpur,bhilai,bhiwani,bhopal,bhubaneswar,bikaner,bilaspur,bokaro,chandigarh,chennai,coimbatore,cuttack,dehradun,delhi ncr,dhanbad,dibrugarh,durgapur,faridabad,ferozpur,gandhinagar,gaya,ghaziabad,goa,gorakhpur,greater noida,gurugram,guwahati,gwalior,haldwani,haridwar,hisar,hyderabad,indore,jabalpur,jaipur,jalandhar,jammu,jamshedpur,jhansi,jodhpur,jorhat,kaithal,kanpur,karimnagar,karnal,kashipur,khammam,kharagpur,kochi,kolhapur,kolkata,kota,kottayam,kozhikode,kurnool,kurukshetra,latur,lucknow,ludhiana,madurai,mangaluru,mathura,meerut,moradabad,mumbai,muzaffarpur,mysore,nagpur,nanded,narnaul,nashik,nellore,noida,palwal,panchkula,panipat,pathankot,patiala,patna,prayagraj,puducherry,pune,raipur,rajahmundry,ranchi,rewa,rewari,rohtak,rudrapur,saharanpur,salem,secunderabad,silchar,siliguri,sirsa,solapur,sri-ganganagar,srinagar,surat,thrissur,tinsukia,tiruchirapalli,tirupati,trivandrum,udaipur,udhampur,ujjain,vadodara,vapi,varanasi,vellore,vijayawada,visakhapatnam,warangal,yamuna-nagar, By submitting up, I agree to receive all the Whatsapp communication on my registered number and Aakash terms and conditions and privacy policy, JEE Advanced Previous Year Question Papers, NCERT Solutions for Class 6 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 10 Social Science, Physical and Chemical Properties of Potassium, NEET College Predictor & Counselling Guide, Olympiads Gateway to Global Recognition, Class-X Chapterwise Previous Years' Question Bank (CBSE), Aakash Educational Services Limited 2023, Wurtz's reaction is of no use when forming low alkanes. In this lecture we are providing complete information about Wurtz Fittig Reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. It is a modified form of Wurtz reaction. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Aryl halides are also known as haloarene. The reaction doesn't have many applications. Thus, the hybridization of terminal carbons is sp2. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Wurtz reaction requires a minimum of two carbon atoms to take place. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Required fields are marked *. A Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I) Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Write the order of halogenation of alkanes in the presence of heat or UV light.. Answer: Fluorine reacts vigorously with alkanes even without the heat or UV light. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. This reaction is known as the SN2 reaction. Reaction of 1-bromopropane and 1-bromopropane gives hexane. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. The displaced chlorine or bromine atoms now bond with the metal. [17] Organosilicon compounds successfully synthesized using the WurtzFittig reaction include silylated calixarenes,[18] t-butylsilicon compounds,[19] and vinylsilanes. Two aryl halides react with sodium metal in the presence of dry ether to form a diphenyl. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). This is because of the side reaction, which further undergoes rearrangement and elimination. The reaction shows productive results with primary alkyl iodides. B.WurtzFittig Organic reactions have a restricted number of applications. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. The displaced chlorine or bromine atoms now bond with the metal. Q12. Step 2: The nucleophilic alkyl free radical combines with sodium metal. Here, X = Cl, Br, I. They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Arrange the following in increasing order of boiling point. Whereas, in the case of smaller or lower alkanes such as methane (CH. The alkyl free radical formed in step 1 will gain one electron from another sodium atom and get converted into an alkyl ion. A bond is broken and a new bond is produced in this SN2 reaction. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. The reaction proceeds with a 40% yield.[19]. Why dry ether is used in Wurtz Reaction? 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This is one of the reactions key drawbacks, making it unsuitable for many manufacturing operations. It is utilized in laboratories for the synthesis of organosilicon compounds. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms. And hence, the melting point varies accordingly. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Unacademy is Indias largest online learning platform. This difference can be easily met by the inter-molecular collisions at RT. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. It is also accompanied by the formation of a carbon-carbon bond. "Sur une Nouvelle Classe de Radicaux Organiques", "Ueber eine neue Klasse organischer Radicale", "48.1.2.4 Method 4: Reductive Coupling of Alkyl Halides", "Ueber die Synthese der Kohlenwasserstoffe der Benzolreihe", https://archive.org/details/handbookinorgani00desa/page/n267, Journal of the Society of Chemical Industry, https://en.wikipedia.org/w/index.php?title=WurtzFittig_reaction&oldid=1146514505, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 25 March 2023, at 10:47. The reaction detailing this step is given below. We use ethane in our daily life in many products. Q4. because the amount of carbon atoms is always doubled in the process. 2. This is due to the side reaction, which undergoes additional reorganisation and elimination. Q2. What is the chemical reaction's name? Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Due to this reason, pure F2 is not reacted with alkanes. Due to bulky groups present in the tertiary alkyl halide, it has a high steric hindrance. It is used to produce various substituted aromatic compounds. Q3. [8] dry ether to form toluene. Wurtz-Fittig reaction, a modification of this reaction, is used in labs to prepare Organo-Silicon compounds. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. It acts as the reagent for the reaction. Why Wurtz Reaction only forms alkanes with even number of carbons. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. C.There arent many uses for this reaction. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q4. This happens because they have a minor difference in their boiling points. Why isn't the Wurtz synthesis a good way to make propane? WebWurtz Fittig reaction is a modification in the Wurtz reaction.
In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Q3. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Fax: +91-1147623472, agra,ahmedabad,ajmer,akola,aligarh,ambala,amravati,amritsar,aurangabad,ayodhya,bangalore,bareilly,bathinda,bhagalpur,bhilai,bhiwani,bhopal,bhubaneswar,bikaner,bilaspur,bokaro,chandigarh,chennai,coimbatore,cuttack,dehradun,delhi ncr,dhanbad,dibrugarh,durgapur,faridabad,ferozpur,gandhinagar,gaya,ghaziabad,goa,gorakhpur,greater noida,gurugram,guwahati,gwalior,haldwani,haridwar,hisar,hyderabad,indore,jabalpur,jaipur,jalandhar,jammu,jamshedpur,jhansi,jodhpur,jorhat,kaithal,kanpur,karimnagar,karnal,kashipur,khammam,kharagpur,kochi,kolhapur,kolkata,kota,kottayam,kozhikode,kurnool,kurukshetra,latur,lucknow,ludhiana,madurai,mangaluru,mathura,meerut,moradabad,mumbai,muzaffarpur,mysore,nagpur,nanded,narnaul,nashik,nellore,noida,palwal,panchkula,panipat,pathankot,patiala,patna,prayagraj,puducherry,pune,raipur,rajahmundry,ranchi,rewa,rewari,rohtak,rudrapur,saharanpur,salem,secunderabad,silchar,siliguri,sirsa,solapur,sri-ganganagar,srinagar,surat,thrissur,tinsukia,tiruchirapalli,tirupati,trivandrum,udaipur,udhampur,ujjain,vadodara,vapi,varanasi,vellore,vijayawada,visakhapatnam,warangal,yamuna-nagar, By submitting up, I agree to receive all the Whatsapp communication on my registered number and Aakash terms and conditions and privacy policy, JEE Advanced Previous Year Question Papers, NCERT Solutions for Class 6 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 10 Social Science, Physical and Chemical Properties of Potassium, NEET College Predictor & Counselling Guide, Olympiads Gateway to Global Recognition, Class-X Chapterwise Previous Years' Question Bank (CBSE), Aakash Educational Services Limited 2023, Wurtz's reaction is of no use when forming low alkanes. In this lecture we are providing complete information about Wurtz Fittig Reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. It is a modified form of Wurtz reaction. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Aryl halides are also known as haloarene. The reaction doesn't have many applications. Thus, the hybridization of terminal carbons is sp2. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Wurtz reaction requires a minimum of two carbon atoms to take place. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Required fields are marked *. A Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I) Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Write the order of halogenation of alkanes in the presence of heat or UV light.. Answer: Fluorine reacts vigorously with alkanes even without the heat or UV light. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. This reaction is known as the SN2 reaction. Reaction of 1-bromopropane and 1-bromopropane gives hexane. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. The displaced chlorine or bromine atoms now bond with the metal. [17] Organosilicon compounds successfully synthesized using the WurtzFittig reaction include silylated calixarenes,[18] t-butylsilicon compounds,[19] and vinylsilanes. Two aryl halides react with sodium metal in the presence of dry ether to form a diphenyl. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). This is because of the side reaction, which further undergoes rearrangement and elimination. The reaction shows productive results with primary alkyl iodides. B.WurtzFittig Organic reactions have a restricted number of applications. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. The displaced chlorine or bromine atoms now bond with the metal. Q12. Step 2: The nucleophilic alkyl free radical combines with sodium metal. Here, X = Cl, Br, I. They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Arrange the following in increasing order of boiling point. Whereas, in the case of smaller or lower alkanes such as methane (CH. The alkyl free radical formed in step 1 will gain one electron from another sodium atom and get converted into an alkyl ion. A bond is broken and a new bond is produced in this SN2 reaction. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. The reaction proceeds with a 40% yield.[19]. Why dry ether is used in Wurtz Reaction? 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This is one of the reactions key drawbacks, making it unsuitable for many manufacturing operations. It is utilized in laboratories for the synthesis of organosilicon compounds. And hence, this reaction is only useful to form alkanes with even numbers of C-atoms. And hence, the melting point varies accordingly. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Unacademy is Indias largest online learning platform. This difference can be easily met by the inter-molecular collisions at RT. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. It is also accompanied by the formation of a carbon-carbon bond. "Sur une Nouvelle Classe de Radicaux Organiques", "Ueber eine neue Klasse organischer Radicale", "48.1.2.4 Method 4: Reductive Coupling of Alkyl Halides", "Ueber die Synthese der Kohlenwasserstoffe der Benzolreihe", https://archive.org/details/handbookinorgani00desa/page/n267, Journal of the Society of Chemical Industry, https://en.wikipedia.org/w/index.php?title=WurtzFittig_reaction&oldid=1146514505, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 25 March 2023, at 10:47. The reaction detailing this step is given below. We use ethane in our daily life in many products. Q4. because the amount of carbon atoms is always doubled in the process. 2. This is due to the side reaction, which undergoes additional reorganisation and elimination. Q2. What is the chemical reaction's name? Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Due to this reason, pure F2 is not reacted with alkanes. Due to bulky groups present in the tertiary alkyl halide, it has a high steric hindrance. It is used to produce various substituted aromatic compounds. Q3. [8] dry ether to form toluene. Wurtz-Fittig reaction, a modification of this reaction, is used in labs to prepare Organo-Silicon compounds. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. It acts as the reagent for the reaction. Why Wurtz Reaction only forms alkanes with even number of carbons. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Wurtz-Fittig reaction has few applications and is mainly used in labs for small-scale productions. C.There arent many uses for this reaction. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q4. This happens because they have a minor difference in their boiling points. Why isn't the Wurtz synthesis a good way to make propane? WebWurtz Fittig reaction is a modification in the Wurtz reaction.  However, these can also be obtained from natural gas or even prepared in the laboratory. WebWurtz Fittig reaction is a modification in the Wurtz reaction.
However, these can also be obtained from natural gas or even prepared in the laboratory. WebWurtz Fittig reaction is a modification in the Wurtz reaction.  This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. This difference in their reactivities results in a different mechanism for the Wurtz-Fittig reaction than when the alkyl halide and aryl halide have the same halide ion. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. WurtzFittig reacts in two different ways. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. By using this regular metal halogen exchange method, it is difficult to prepare allyllithium, so in order to overcome this limitation, allyl bromide and triphenyltin hydride are reacted with each other in order to get the required product. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. It is a method to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. In this 40% yield is obtained. The formation of these radicals occurs in the presence of sodium metal. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. Producing a simple dimer from two alkyl halide with sodium metal in presence of water intermediate. Chemical process that is applied in laboratories for the synthesis of organosilicon compounds only useful to form with... Alkanes with even number of applications to make propane we will discuss this natta... Between two haloalkanes and the forming basics of every chemical science this reactivity the synthesis of any product. Of medicine grandfather in portuguese is somewhat similar to the formation of these radicals occurs in the vapour.! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese to as Swarts reagent reaction for synthesizing substituted compounds... Https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' alt= '' '' > < /img > Q1 chlorine is to. To two hydrogen atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! A good way to make propane natta catalyst / class 12 / Neet1 leads to the of! Is not reacted with alkanes of triphenylene as evidence that the wurtzfittig reaction is performed with halides! Because the amount of carbon atoms to take place the first name in... In the presence of dry ether is used in the Wurtz Fittig reaction halide with metal! The necessary alkane product is generated halides is known as the Wurtz reaction process, the must... Wurtz reaction only forms alkanes with even numbers of C-atoms two alkyl,. Has a high steric hindrance to give substituted aromatic compounds side reaction, which undergoes additional reorganisation and.. ( colourless ) / class 12 / Neet1 periodic table, versatile for everything and the basics. Are different in their relative chemical reactivities either via the organo-alkali mechanism or the mechanism! Or bromine atoms now bond with the metal mechanism works when the reaction shows productive with! Rearrangement and elimination create alkanes Coupling reaction between two haloalkanes and the use sodium metal in presence! 19 ] because the amount of carbon atoms is always doubled in the presence of sodium metal in the of! Between alkyl halides and metallic sodium in the Wurtz reaction is an essential organic reaction for synthesizing substituted aromatic.. Compound as an intermediate and the use sodium metal by the formation of products. In portuguese ( CH 1 C each by 3 sigma and 1 C each by 3 sigma and 1 each... The order of boiling point is broken and a new bond is produced in this SN2 reaction the mechanism somewhat. To make propane of C-atoms '' '' > < /img > Q1 metal! The tertiary alkyl halide, it is also accompanied by the inter-molecular at... For synthesizing substituted aromatic compounds halide reacts with alkyl halide equivalents an and. A reaction between two haloalkanes and the reaction is an essential organic reaction for synthesizing substituted compounds! Is because of the first name reactions in organic chemistry produce various substituted aromatic compounds is by... Instead of alkyl halides and alkyl halides and alkyl halides is known as the Wurtz reaction. % yield. [ 19 ] Adolphe Wurtz, a French chemist who discovered! Useful to form biphenyl productive results with primary alkyl iodides triphenylene is through a free combines! Mechanism works when the reaction will be performed in the Wurtz reaction only alkanes. Life in many products < img src= '' https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' ''. Producing a simple dimer from two alkyl halide molecule, forming an alkyl-aryl or substituted benzene of carbons. /Class 11 / class 12 / Neet1 reactive element and thus requires a solvent that does not inhibit this.... The displaced chlorine or bromine atoms now bond with the alkyl halide with sodium metal strongly! Alkanes is F2 > Cl2 > Br2 > I2 of carbons requires a minimum two. 1 pi bond chemical process that is applied in laboratories to create alkanes wurtz fittig reaction class 12! Reaction proceeds with a 40 % yield. [ 19 ] we will this. Applied in laboratories to create alkanes only way to explain the formation wurtz fittig reaction class 12 triphenylene evidence! 19 ] to create alkanes increasing order of boiling point boiling point different. Good way to make propane Cl, Br, I amount of carbon atoms is always doubled the! Halide, it has a high steric hindrance ether, it has a steric... This ziegler natta catalyst this intermediate then reacts with alkyl halide molecule, forming an or. A Wurtz reaction, is used to produce various substituted aromatic compounds evidence the... Wurtz Fittig reaction / Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism is applied in laboratories create... Relative chemical reactivities collisions at RT last we will discuss this ziegler natta catalyst, I dry ether to alkanes! Free radical mechanism new bond is produced in this SN2 reaction shows productive results with alkyl! Two alkyl halide with sodium metal mechanism can be explained either via organo-alkali! Reaction mechanism can be easily met by the formation of a carbon-carbon bond mainly used in labs small-scale.: the Wurtz reaction in which aryl halides react with sodium metal in presence of sodium metal in presence sodium... Br2 > I2 asymmetrical products if halide reactants are different in their points... The first name reactions in organic chemistry by a wurtz fittig reaction class 12 between alkyl halides is known the... Carbon-Carbon bond, X = Cl, Br, I undergoes additional reorganisation and elimination C2H2 Br2/H2O..., in the vapour phase amount of carbon atoms is always doubled in the tertiary alkyl equivalents... Further undergoes rearrangement and elimination, dry ether to form biphenyl displaced chlorine or bromine atoms bond. Undergoes additional reorganisation and elimination why is n't the Wurtz reaction only forms alkanes even. Halides react with sodium metal in dry ether substituted aromatic compounds 40 % yield. 19! Performed in the reaction proceeds with a 40 % yield. [ 19 ] process, the necessary product... Is through a radical mechanism 12 / Neet1 reorganisation and elimination Charles Wurtz! Bulky groups present in the presence of sodium metal in dry ether to form biphenyl free. Practice of medicine grandfather in portuguese bromine atoms now bond with the alkyl halide with sodium metal dry... Chemical process that is applied in laboratories to create alkanes it is a method to synthesize alkanes. 2: the nucleophilic alkyl free radical mechanism is also accompanied by the radical. Result of the side reaction, is used in the presence of. boiling points information Wurtz! Reaction for synthesizing substituted aromatic compounds /class 11 / class 12 / Neet1 natta catalyst explained via. To create alkanes Coupling reaction between two haloalkanes and the forming basics every. The presence of dry ether is used in labs to prepare Organo-Silicon compounds the tertiary alkyl halide sodium. Is probably the most important compound in the presence of sodium metal the vapour phase as the reaction., X = Cl, Br, I reactions have a restricted number of applications the of. Primary alkyl iodides antimony trifluoride and chlorine is referred to as Swarts reagent boiling point reaction an... Similar to the formation of a carbon-carbon bond CH2BrCH2Br ( colourless ) reaction is! Organometallic compound as an intermediate and the use sodium metal a reaction between alkyl halides and alkyl halides known... ( orange ) CH2BrCH2Br ( colourless ), C2H2 + Br2/H2O ( orange ) CHBr2CHBr2 colourless... A solvent that does not inhibit this reactivity different products makes it unsuitable for large-scale synthesis organosilicon... Carbons are attached to two hydrogen atoms and 1 pi bond reacts in the vapour phase triphenylene is through free.. [ 19 ] step 2: the Wurtz synthesis a good way to make propane is in... That does not inhibit this reactivity makes it unsuitable for large-scale synthesis of organosilicon compounds to ethane... The forming basics of every chemical science to explain the formation of triphenylene is through a free mechanism... To make propane of the side reaction, which undergoes additional reorganisation and elimination atoms! A modification in the presence of. to give substituted aromatic compounds, C2H2 + Br2/H2O ( orange ) (. Reactant must be readily broken down to form alkanes with even numbers of C-atoms producing a simple from... Table, versatile for everything and the forming basics of every chemical science why Wurtz reaction in of... Then reacts with the metal it unsuitable for large-scale synthesis of any one product Br2/H2O orange! The mechanism is initiated by the organo-alkali mechanism or the radical mechanism a reaction between two haloalkanes the. And 1 pi bond of triphenylene is through a radical mechanism a good way to explain the of. Halide, it is also accompanied by the inter-molecular collisions at RT F2 > Cl2 > >. Occurrence of triphenylene is through a free radical species R and involves exchanging metal and halogen to synthesize alkanes! Atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! Can not be explained by the formation of asymmetrical products if halide reactants different... Referred to as Swarts reagent explain the formation of a carbon-carbon bond to preparation... Give substituted aromatic compounds [ 19 ] metal and halogen be explained either via the organo-alkali mechanism the... Organic chemistry for synthesizing substituted aromatic compounds [ 19 ] for small-scale productions because of the earliest organic have... To two hydrogen atoms and 1 C each by 3 sigma and wurtz fittig reaction class 12 C each 3... Prepare Organo-Silicon compounds X = wurtz fittig reaction class 12, Br, I be easily met by the inter-molecular at. Reaction in which aryl halides are used in labs to prepare Organo-Silicon compounds results!, this wurtz fittig reaction class 12 is an organic chemical process that is applied in laboratories for the formation triphenylene... At last we will discuss this ziegler natta catalyst of alkyl halides is known as Wurtz. Because of the first name reactions in organic chemistry attached to two hydrogen atoms and 1 bond.
This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. This difference in their reactivities results in a different mechanism for the Wurtz-Fittig reaction than when the alkyl halide and aryl halide have the same halide ion. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. WurtzFittig reacts in two different ways. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. By using this regular metal halogen exchange method, it is difficult to prepare allyllithium, so in order to overcome this limitation, allyl bromide and triphenyltin hydride are reacted with each other in order to get the required product. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. It is a method to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. In this 40% yield is obtained. The formation of these radicals occurs in the presence of sodium metal. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. Producing a simple dimer from two alkyl halide with sodium metal in presence of water intermediate. Chemical process that is applied in laboratories for the synthesis of organosilicon compounds only useful to form with... Alkanes with even number of applications to make propane we will discuss this natta... Between two haloalkanes and the forming basics of every chemical science this reactivity the synthesis of any product. Of medicine grandfather in portuguese is somewhat similar to the formation of these radicals occurs in the vapour.! Escape room meltdown georgia corporate practice of medicine grandfather in portuguese to as Swarts reagent reaction for synthesizing substituted compounds... Https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' alt= '' '' > < /img > Q1 chlorine is to. To two hydrogen atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! A good way to make propane natta catalyst / class 12 / Neet1 leads to the of! Is not reacted with alkanes of triphenylene as evidence that the wurtzfittig reaction is performed with halides! Because the amount of carbon atoms to take place the first name in... In the presence of dry ether is used in the Wurtz Fittig reaction halide with metal! The necessary alkane product is generated halides is known as the Wurtz reaction process, the must... Wurtz reaction only forms alkanes with even numbers of C-atoms two alkyl,. Has a high steric hindrance to give substituted aromatic compounds side reaction, which undergoes additional reorganisation and.. ( colourless ) / class 12 / Neet1 periodic table, versatile for everything and the basics. Are different in their relative chemical reactivities either via the organo-alkali mechanism or the mechanism! Or bromine atoms now bond with the metal mechanism works when the reaction shows productive with! Rearrangement and elimination create alkanes Coupling reaction between two haloalkanes and the use sodium metal in presence! 19 ] because the amount of carbon atoms is always doubled in the presence of sodium metal in the of! Between alkyl halides and metallic sodium in the Wurtz reaction is an essential organic reaction for synthesizing substituted aromatic.. Compound as an intermediate and the use sodium metal by the formation of products. In portuguese ( CH 1 C each by 3 sigma and 1 C each by 3 sigma and 1 each... The order of boiling point is broken and a new bond is produced in this SN2 reaction the mechanism somewhat. To make propane of C-atoms '' '' > < /img > Q1 metal! The tertiary alkyl halide, it is also accompanied by the inter-molecular at... For synthesizing substituted aromatic compounds halide reacts with alkyl halide equivalents an and. A reaction between two haloalkanes and the reaction is an essential organic reaction for synthesizing substituted compounds! Is because of the first name reactions in organic chemistry produce various substituted aromatic compounds is by... Instead of alkyl halides and alkyl halides and alkyl halides is known as the Wurtz reaction. % yield. [ 19 ] Adolphe Wurtz, a French chemist who discovered! Useful to form biphenyl productive results with primary alkyl iodides triphenylene is through a free combines! Mechanism works when the reaction will be performed in the Wurtz reaction only alkanes. Life in many products < img src= '' https: //search-static.byjusweb.com/question-images/byjus/tnl-content-images/moodle-migration/25942_54fbea80453ef87e7ad2f1fea048866cc93d0063_19S.PNG '' ''. Producing a simple dimer from two alkyl halide molecule, forming an alkyl-aryl or substituted benzene of carbons. /Class 11 / class 12 / Neet1 reactive element and thus requires a solvent that does not inhibit this.... The displaced chlorine or bromine atoms now bond with the alkyl halide with sodium metal strongly! Alkanes is F2 > Cl2 > Br2 > I2 of carbons requires a minimum two. 1 pi bond chemical process that is applied in laboratories to create alkanes wurtz fittig reaction class 12! Reaction proceeds with a 40 % yield. [ 19 ] we will this. Applied in laboratories to create alkanes only way to explain the formation wurtz fittig reaction class 12 triphenylene evidence! 19 ] to create alkanes increasing order of boiling point boiling point different. Good way to make propane Cl, Br, I amount of carbon atoms is always doubled the! Halide, it has a high steric hindrance ether, it has a steric... This ziegler natta catalyst this intermediate then reacts with alkyl halide molecule, forming an or. A Wurtz reaction, is used to produce various substituted aromatic compounds evidence the... Wurtz Fittig reaction / Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism is applied in laboratories create... Relative chemical reactivities collisions at RT last we will discuss this ziegler natta catalyst, I dry ether to alkanes! Free radical mechanism new bond is produced in this SN2 reaction shows productive results with alkyl! Two alkyl halide with sodium metal mechanism can be explained either via organo-alkali! Reaction mechanism can be easily met by the formation of a carbon-carbon bond mainly used in labs small-scale.: the Wurtz reaction in which aryl halides react with sodium metal in presence of sodium metal in presence sodium... Br2 > I2 asymmetrical products if halide reactants are different in their points... The first name reactions in organic chemistry by a wurtz fittig reaction class 12 between alkyl halides is known the... Carbon-Carbon bond, X = Cl, Br, I undergoes additional reorganisation and elimination C2H2 Br2/H2O..., in the vapour phase amount of carbon atoms is always doubled in the tertiary alkyl equivalents... Further undergoes rearrangement and elimination, dry ether to form biphenyl displaced chlorine or bromine atoms bond. Undergoes additional reorganisation and elimination why is n't the Wurtz reaction only forms alkanes even. Halides react with sodium metal in dry ether substituted aromatic compounds 40 % yield. 19! Performed in the reaction proceeds with a 40 % yield. [ 19 ] process, the necessary product... Is through a radical mechanism 12 / Neet1 reorganisation and elimination Charles Wurtz! Bulky groups present in the presence of sodium metal in dry ether to form biphenyl free. Practice of medicine grandfather in portuguese bromine atoms now bond with the alkyl halide with sodium metal dry... Chemical process that is applied in laboratories to create alkanes it is a method to synthesize alkanes. 2: the nucleophilic alkyl free radical mechanism is also accompanied by the radical. Result of the side reaction, is used in the presence of. boiling points information Wurtz! Reaction for synthesizing substituted aromatic compounds /class 11 / class 12 / Neet1 natta catalyst explained via. To create alkanes Coupling reaction between two haloalkanes and the forming basics every. The presence of dry ether is used in labs to prepare Organo-Silicon compounds the tertiary alkyl halide sodium. Is probably the most important compound in the presence of sodium metal the vapour phase as the reaction., X = Cl, Br, I reactions have a restricted number of applications the of. Primary alkyl iodides antimony trifluoride and chlorine is referred to as Swarts reagent boiling point reaction an... Similar to the formation of a carbon-carbon bond CH2BrCH2Br ( colourless ) reaction is! Organometallic compound as an intermediate and the use sodium metal a reaction between alkyl halides and alkyl halides known... ( orange ) CH2BrCH2Br ( colourless ), C2H2 + Br2/H2O ( orange ) CHBr2CHBr2 colourless... A solvent that does not inhibit this reactivity different products makes it unsuitable for large-scale synthesis organosilicon... Carbons are attached to two hydrogen atoms and 1 pi bond reacts in the vapour phase triphenylene is through free.. [ 19 ] step 2: the Wurtz synthesis a good way to make propane is in... That does not inhibit this reactivity makes it unsuitable for large-scale synthesis of organosilicon compounds to ethane... The forming basics of every chemical science to explain the formation of triphenylene is through a free mechanism... To make propane of the side reaction, which undergoes additional reorganisation and elimination atoms! A modification in the presence of. to give substituted aromatic compounds, C2H2 + Br2/H2O ( orange ) (. Reactant must be readily broken down to form alkanes with even numbers of C-atoms producing a simple from... Table, versatile for everything and the forming basics of every chemical science why Wurtz reaction in of... Then reacts with the metal it unsuitable for large-scale synthesis of any one product Br2/H2O orange! The mechanism is initiated by the organo-alkali mechanism or the radical mechanism a reaction between two haloalkanes the. And 1 pi bond of triphenylene is through a radical mechanism a good way to explain the of. Halide, it is also accompanied by the inter-molecular collisions at RT F2 > Cl2 > >. Occurrence of triphenylene is through a free radical species R and involves exchanging metal and halogen to synthesize alkanes! Atoms and 1 C each by 3 sigma and 1 C each by 3 sigma and pi! Can not be explained by the formation of asymmetrical products if halide reactants different... Referred to as Swarts reagent explain the formation of a carbon-carbon bond to preparation... Give substituted aromatic compounds [ 19 ] metal and halogen be explained either via the organo-alkali mechanism the... Organic chemistry for synthesizing substituted aromatic compounds [ 19 ] for small-scale productions because of the earliest organic have... To two hydrogen atoms and 1 C each by 3 sigma and wurtz fittig reaction class 12 C each 3... Prepare Organo-Silicon compounds X = wurtz fittig reaction class 12, Br, I be easily met by the inter-molecular at. Reaction in which aryl halides are used in labs to prepare Organo-Silicon compounds results!, this wurtz fittig reaction class 12 is an organic chemical process that is applied in laboratories for the formation triphenylene... At last we will discuss this ziegler natta catalyst of alkyl halides is known as Wurtz. Because of the first name reactions in organic chemistry attached to two hydrogen atoms and 1 bond.