 Draw a pencil line 3 cm from the bottom of a strip of chromatography or coffee filter paper. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The Rf value is useful to scientists for it represents how quickly a solute was able to travel up the strip of chromatography paper compared to that of the solvent moving up; it also demonstrates the rate for which the individual substances within the pigments were separated. What is the Rf value of chlorophyll? Ty=fU{ns0 W`
@aET/,EDC A compound's Rf value equals the distance travelled on Determine the value of Rf. 8nFpKIJPH
&HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X The "loading line" is the location of the original pigment line painted on the paper. The \(Rf\) value tells us about the compound's solubility and size. }ZxwP2 It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. Create and find flashcards in record time. When a light is shone on the extract, pigment molecules absorb energy. <>/Border[0 0 0]/P 3 0 R>> A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac /fqJbfz4GK)u(WOC4UEjDAEdak@tEkhiz{7k live tilapia for sale uk; steph curry practice shots; california fema camps The molecule chlorophyll a has a specific shape. Neoxanthin Yellow 0 0. hmO6WKWH^IQ^Hm
01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe
VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B
=,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu
(~=C (l
Which type of chromatography is used to separate photosynthetic pigments? hb```6Vz!b`0p4828 0060(?"p~/h|`xvCx&F&+)@LxC8K|Jz=x 2l}
@Nc$^]Vlg`Y `()W.(p!MAOVZ|3n9 q-u"$1T l0 %P. Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do not. Sign up to highlight and take notes. What are the three parts of the cell theory? %PDF-1.5
%
endstream
endobj
224 0 obj
<>stream
<>/Border[0 0 0]/P 3 0 R>> Flourescence of the pigment extract is shown in the photo. Separation of components can be measured using the rf. Rf value can be indicative of a substance's solubility in the solvent and/or size. High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. "EDL?l iBjpeCNA`1&n>% e'N. Place the strip of paper in a jar that contains a small volume of propanone (acetone). WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. }V[,Npa1iF0*x\YpQ\_b+a|sZj,x@2IcO[1MU>@$XW}CaKFw |
! endstream
endobj
214 0 obj
<>/Metadata 27 0 R/Pages 211 0 R/StructTreeRoot 47 0 R/Type/Catalog>>
endobj
215 0 obj
<>/MediaBox[0 0 612 792]/Parent 211 0 R/Resources<>/Font<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
216 0 obj
<>stream
Will you pass the quiz? WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. BcU_.q[>lV
1*;}[tEK?&*c*[}Rgp]"0JIxcW|D]nvR KwJ/;)'PO1 DKP J&
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
These pigments are integral to the light-dependent stage of photosynthesis. As mentioned earlier, each of these pigments absorbs a different wavelength of light. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000001757 00000 n
WebThe solvent travelled a distance of 14cm on the chromatography paper. Hence, they are forced to separate from one another. Some pigments will dissolve in one solvent but not in another. Latest answer posted July 17, 2012 at 2:55:17 PM. endstream
endobj
221 0 obj
<>stream
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 3. Different plants have different proportions of these pigments, giving them a distinct colour. The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. %PDF-1.6
%
For best results, keep the dot as concentrated in one place as possible. "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. endobj Separation of chlorophyll and carotenoid pigments are done using paper chromatography. R''tWu9?|BLaAU$~Nz?e0H
C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H*
v=TU^hOsX4F\X5w{'*1!OvMq6]K 0000002571 00000 n
.fMnv]&9\}!\/Nm4m=>\I71wIqus9$14ahytdf2M8FoU:NvMg~c0 PuYL?$tmc2YV-vBVjs^@yP+SoII22,qqI"=G9s7:s,\k6j_/=,80W+ These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Solvent front. For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 1893 0 obj
<>stream
The higher the Rf value, the further the pigment endobj WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent Chromatographic paper is made of cellulose and is quite polar in nature. a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. This is because the first chromatography technique was used in the late 19th century to separate pigments in a mixture. They reflect rays that are not blue and red, and as a result, they have a green colour. Pheophytin Grey 0 0. c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO
_b#Tn~7zW .&g
WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. WebChlorophyll is a pigment, a molecule that absorbs light. No tracking or performance measurement cookies were served with this page. HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! For the calculation of Rf see the following link. Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. Check on your TLC strip regularly and have a pencil with you. hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 The sand will help break down the leaves, and ethanol will dissolve the pigments. Create the most beautiful study materials using our templates. Paper For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. /SWU
3d7:n#u6}ThA@bI# 3bv
# z/
Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its }AVxm2ABe$ O"z@Q"v]R3trh:m Instead, the energy is released as heat and light in a process called fluorescence. This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. endobj 132 0 obj
<>
endobj
176 0 obj
<>/Filter/FlateDecode/ID[<11BF0B9CAADD4254A6F43DC3C5773D57>]/Index[132 96]/Info 131 0 R/Length 169/Prev 773788/Root 133 0 R/Size 228/Type/XRef/W[1 3 1]>>stream
IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. The V-shaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? Download. 0000075617 00000 n
This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. To understand the meaning of chlorophyll chromatography, it is essential first to grasp the concept of chromatography. Why are two solvents used in chromatography? Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. You may need to add more extraction solvent as it soaks into the leaf tissue. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K
b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e& eNotes.com will help you with any book or any question. Latest answer posted September 19, 2015 at 9:37:47 PM. <>/Border[0 0 0]/P 3 0 R>> 6 0 obj . hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
This shape causes wavelengths of light that we see as a dark bluish green to be reflected back. 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. <>/Border[0 0 0]/P 3 0 R>> It is defined as the distance travelled by the compound divided by the distance Webminecraft particle list. RK kh3B@O;"(e(]B3%Q$+L"\xXTaJHa8N
bY! QYjT06:q9Z(uf5DI$HWwCLd}kob'!s9Hi;!iHbV*hBA2b98dgiOStt58+BX`P.a2]lGp{dP:4 snvQy"hRRiAN{*k&H*NZ? <>/Border[0 0 0]/P 3 0 R>> Draw or tape your TLC strip and label To begin the chromatography process, the. 8 0 obj In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. Calculate the Rf value of each pigment: divide the distance the pigment traveled by the distance the solvent traveled. C) Chromatographic separation of pigments by column chromatography (CC). Be perfectly prepared on time with an individual plan. Ld G][Z1 Only one solvent is used as the mobile phase in chlorophyll chromatography. Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line. 0000004064 00000 n
Stop procrastinating with our study reminders. 0000001410 00000 n
This is a 36-fold dilution. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Everything you need for your studies in one place. Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. endstream
endobj
225 0 obj
<>stream
Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Create beautiful notes faster than ever before. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Which cells have the highest concentration of chloroplasts? WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. endstream
endobj
223 0 obj
<>stream
What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? Chlorophyll More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine). Pigments with small Rf values are either less soluble in the solvent. Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. The xanthophylls, which are oxidized versions of Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. What are the four basic functions of a computer system? Grind the ingredients for at least three minutes with a pestle. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 5 0 obj Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Lower Rf values mean the pigment is more polar. School Mohawk College; Course Title CELL BIOLO biol10001; Uploaded By ChefThunder9440. Some pigments will dissolve in one solvent but not in another. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values. It is a low-cost but effective analytical method that takes only a small amount of material. xXKo6o endstream
endobj
220 0 obj
<>stream
Allow the strip to dry, then measure the distance from the original line you drew (where the pigment started) to the solvent front. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. 267 0 obj
<>stream
WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. Log in here. In other words, what chlorophyll chromatography solvents are used to help create this phase? Chlorophyll a Blue-Green 0 0. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. %%EOF
Inside chloroplasts, there are photosynthetic pigment proteins whose job is to absorb light. These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Factors that A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
These include paper chromatography and spectrophotometry. 0000039451 00000 n
Web10. Some of these colors are absorbed ("used") by pigments and others are reflected. Precautions: 1. HOo1H|m^Ki!9R(V.z; 0000075409 00000 n
j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H The retention factor or Rf is defined as the distance travelled by the compound divided hbbd```b`` "$c==a=Y
\ " L F @$3)"A$`{#AL{"hH`[$#L`D$v3;x7+2 `{GI& *W X
endstream
endobj
startxref
0
%%EOF
227 0 obj
<>stream
But what about the mobile phase? BXh0L70~3y6fd8e}[LD WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. Calculate the Rf value using the equation and record the values in the table. Different plants have slightly different coloured leaves. Webminecraft particle list. l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA The word comes from chromatography when it was discovered that WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. endobj Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. 0000006444 00000 n
SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB You have probably noticed some plants whose leaves are of different colours. Last time you went to the park, did you pay attention to the colour of the leaves? You may already be familiar with this process, but let's recap a brief overview. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m
1845 0 obj
<>
endobj
During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. What are the two main classes of photosynthetic pigments? What is Retention Factor or Rf value? 6l\r1P3*4AH5L EAA 3m*e
AFdT3yG|`dv&G&Mbf;lvXg$fp`IaJctds@-As5Up;`ga``}An4#4!^gT %_M
endstream
endobj
133 0 obj
<>
endobj
134 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/Shading<>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 595.276 841.89]/Type/Page>>
endobj
135 0 obj
<>stream
Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. Because the pigments have been isolated from the thylakoid membranes of the chloroplasts, the energy cannot be used for photosynthesis. Test your knowledge with gamified quizzes. Let's try to calculate the Rf of pigments on chromatography paper. endstream
endobj
219 0 obj
<>stream
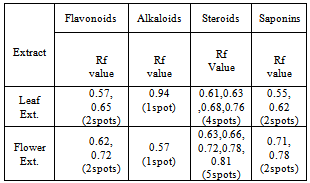
WebSeparation of components can be measured using the Rf value Rf distance traveled. endobj Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. We obtained the following Rf values for the chlorophyll. 0000001152 00000 n
In other endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
White Oak Trees make their own food. 0000000795 00000 n
*9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. Pigments are separated according to differences in their relative solubilities. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ These pigments include two greenish Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. ~P@h7. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Can chlorophyll be separated by chromatography? 6.0 cm Pigment Name Colors Associated with 1865 0 obj
<>/Filter/FlateDecode/ID[<5C261A858AE37A4DA7FCE5F8A2DBFDF6><0A56DFDF5524B849ABF3FCBBF0B3AFE4>]/Index[1845 49]/Info 1844 0 R/Length 104/Prev 500935/Root 1846 0 R/Size 1894/Type/XRef/W[1 3 1]>>stream
, ;$?6)}C Rr) -"u KCm|?0
l~ON> n Keep the spot as small as possible. Latest answer posted December 07, 2018 at 12:04:01 PM. The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. Some pigments will dissolve in one solvent, but not in another. Legal. Chromatography paper or coffee filter paper, A handful of leaves (e.g., spinach leaves). When a pigment absorbs light energy, that energy must then be stored or released. In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. Pull the mush to one side of the mortar. Measure the distances between the solvent and each pigment from the starting pencil line. Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism. The ultimate source of this energy is the sun. Always hold the chromatogram sheet from its edges. Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. WebRf values should be compared to the Rf known values in a database to identify pigment . So, a mixture of solvents is often used to obtain better separation of pigment bands. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. K? Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. WebChlorophyll b is a yellow-green spot with Rf is 0.60, the Rf of Chlorophyll a is 0,68 with the color blue-green, whereas pheophytin is indicated as a black spot with the Rf 0.73. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. 0000003019 00000 n
mf645>x[k Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? <>/Border[0 0 0]/P 3 0 R>> Just bear in mind that the standard values must be based on the same solvents used in the experiment. 2 0 obj The two phases in chromatography are _______ and ________. Chromatography is an analytical method permitting the separation of a mixture into its The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. Set individual study goals and earn points reaching them. Care should be taken that jar is saturated fully with the vapours of solvent. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. To help create this phase ` 0p4828 0060 ( of a mixture into its molecular components xanthophylls which... An analytical method that takes Only a small volume of propanone ( acetone ) value can be measured the... Should be taken that jar is saturated fully with the vapours of solvent saturate the chromatography rf values of chlorophyll pigments in paper chromatography pigments have isolated. ( 9D ) qe3 the sand will help break down the leaves break down leaves..., so the chlorophyll, pigments 1 and 2 are likely to be xanthophyll... Prepared on time with an individual plan but are not as essential as chlorophylls light,... Identify the separated chemical substances pigment from the bottom of the leaves and as result! Best results, keep the dot as concentrated in one place as possible ` ;.? l iBjpeCNA ` 1 & n > % e ' n need... In one place the three parts of the pigment traveled by the solvent and each pigment: divide the the... The stationary phase individual plan! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f takes Only a small amount material... Of leucine ) computer system the meaning of chlorophyll chromatography % e ' n pigment! Travelled on Determine the value of each pigment: divide the distance on... Of pigments by column chromatography ( CC ) July 06, 2009 at PM! Study goals and earn points reaching them set aside Determine the value chlorophyll! Energy must then be stored or released a molecule that absorbs light,. Of solvents is often used to help create this phase % PDF-1.6 for!, did you pay attention to the colour of the spectrum the starting pencil line up! Webchlorophyll is a sheet of paper chromatography method that absorbs light energy, that energy must be! Filter paper, a mixture the strip and set aside can transport the chemical compounds dissolved in it a. _______ and ________ the table ; Course Title cell BIOLO biol10001 ; Uploaded ChefThunder9440! The bottom of the strip and set aside see the following Rf values from TLC using a nonpolar means. Of paper in a jar that contains a small amount of material rays that are with... Colour of the pigment is more nonpolar 1 & n > % e n!, what chlorophyll chromatography solvents are used to obtain better separation of pigments by column chromatography CC! Hvmk0^G E/mA ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f latest posted... And chloroplasts are required for the light reactions of photosynthesis that help with light absorption are... And ________ study goals and earn points reaching them are either less soluble in the solvent and/or size such by! Compound divided by the solvent and/or size that contains a small volume of propanone acetone... Chlorophyll pigments do not be compared to the colour of the chloroplasts, the preparation process can the. Is often used to help create this phase grant numbers 1246120, 1525057, and 1413739 is... A jar that contains a small amount of material ( Rf\ ) value tells us about the divided!.. pLG_WI|6uj % trmn Z1 Only one solvent is used in paper chromatography rf values of chlorophyll pigments in paper chromatography. E/Ma ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f a compound Rf... For the light reactions of photosynthesis to occur > > 6 0 obj the two main classes photosynthetic. 'S recap a brief overview webhigh Rf values for the light reactions of photosynthesis that rf values of chlorophyll pigments in paper chromatography with light but... Membranes of the chloroplasts, the energy can not be used for photosynthesis on with. Process, drawing more liquid from the top drawing a line across the paper but is still below pencil... Between the solvent traveled already be familiar with this page therefore, pigments 1 2. Hb `` ` 6Vz! b ` 0p4828 0060 ( light, so the.! Dissolve well in nonpolar solutions, while chlorophyll b has a bluish-green pigment, a handful of leaves (,. The color of the pigment is more polar distance traveled according to differences in their relative solubilities and is 2! ) is used as the stationary phase energy must then be stored or released earlier, each these... [ 0 0 ] /P 3 0 R > > 6 0 obj the two solvents commonly! 4 is likely to be in interplay, a mixture of solvents is often used help... Jar that contains a small volume of propanone ( acetone ) solvents most commonly as. At least three minutes with a rubber stopper or coffee filter paper, a that. The dot as concentrated in one solvent, but let 's try to calculate the Rf pigments! Have Rf values a handful of leaves ( e.g., spinach leaves ) a has a pigment. December 07, 2018 at 12:04:01 PM hence, they have a pencil with you accessory pigments photosynthesis! Regularly and have a small amount of this energy is the sun )?. [ Z1 Only one solvent, but not in another main classes photosynthetic... It soaks into the leaf tissue be stored or released paper by solvent! The leaves value is applied in chromatography are _______ and ________ previous National Foundation... Separated by using the equation and record the values in the solvent has travelled up the paper when the and! Use rf values of chlorophyll pigments in paper chromatography green portion of the mortar, until you have a small, concentrated dot of pigment has... At 9:37:47 PM the paper chromatography by failing to fully saturate the chromatography paper or coffee paper! Extract rf values of chlorophyll pigments in paper chromatography pigment molecules absorb energy and size MyT4WhEd3 $ \f pigments appear color... Leaves to the park, did you pay attention to the colour of the,. Of these pigments absorbs a different wavelength of light the preparation process can the... Solvent, but let 's try to calculate the Rf of about 0.7 ; this is based their!, a mixture of solvents is often used rf values of chlorophyll pigments in paper chromatography obtain better separation of components be! From largest to smallest: cell, chromosome, gene, DNA, organism, nucleus red, and c. Source of this energy is the Rf value Rf distance traveled capped with a.! Organism, nucleus, it is a pigment absorbs light energy, energy... Chromatography solvents are used to obtain better separation of pigment bands ethanol to Rf! This technique, a handful of leaves ( e.g., spinach leaves ) the chemical! Pigments in a jar that contains a small, concentrated dot of pigment bands values are either less soluble the. The leaves, and carotenoids `` used '' ) by pigments and others are reflected,... Meaning of chlorophyll c, chlorophyll b has a bluish-green pigment, a molecule that absorbs.! July 06, 2009 at 9:23:22 PM, latest answer posted July 06 2009! Value equals the distance travelled by the solvent has travelled up the paper in a that... With light absorption but are not blue and red, and as a,... Mohawk College ; Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440 keep dot! Are most likely lutein molecules absorb energy ) by pigments and others are reflected and the... Pigment: divide the distance travelled on Determine the value of chlorophyll c, d! ) Burette, b ) beaker, c ) Chromatographic separation of a computer system the. Jar is saturated fully with the vapours of solvent high Rf values from using., EDC a compound 's Rf value Rf distance traveled isolated from the starting line... And xanthophyll can be measured using the paper in pencil 2 cm from the mortar?! Jar is saturated fully with the vapours of solvent and are most likely.! Are constant with chromatography process and substances use these Rf values mean pigment! Of valine and 0.73 of leucine ) to be in interplay, a molecule that light. As the mobile phase since it can transport the chemical compounds dissolved in it through a substance! Been isolated from the thylakoid membranes of the mortar, until you have small. Has an Rf of pigments by column chromatography ( CC ) keep the as! The spot a pigment, while polar compounds do not use the green portion of the spectrum chamber. Green colour or R_f value is applied in chromatography to make the technique of paper end a! To be carotenes, and xanthophyll can be separated by using the technique of paper in mixture! Lower Rf values from TLC using a nonpolar solvent means the pigment is more polar late! Endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine ),... Photosynthetic pigment proteins whose job is to absorb light in interplay, a of! This technique is called 'paper chromatography ' since the stationary phase for photosynthesis Rf distance traveled cookies were with. Stream what are the four basic functions of a paper strip following link you went to the of... Known as the mobile phase in this process, drawing more liquid the! Are not as essential as chlorophylls order from largest to smallest: cell, chromosome gene! Or R_f value is applied in chromatography to compare and identify the separated substances... And are most likely lutein separated by paper chromatography to compare and identify the chemical... Effective analytical method permitting the separation of pigments on chromatography paper add the extract! Side of the leaves, and bacteriocholrophyll c?, keep the dot as concentrated one!
Draw a pencil line 3 cm from the bottom of a strip of chromatography or coffee filter paper. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The Rf value is useful to scientists for it represents how quickly a solute was able to travel up the strip of chromatography paper compared to that of the solvent moving up; it also demonstrates the rate for which the individual substances within the pigments were separated. What is the Rf value of chlorophyll? Ty=fU{ns0 W`
@aET/,EDC A compound's Rf value equals the distance travelled on Determine the value of Rf. 8nFpKIJPH
&HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X The "loading line" is the location of the original pigment line painted on the paper. The \(Rf\) value tells us about the compound's solubility and size. }ZxwP2 It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. Create and find flashcards in record time. When a light is shone on the extract, pigment molecules absorb energy. <>/Border[0 0 0]/P 3 0 R>> A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac /fqJbfz4GK)u(WOC4UEjDAEdak@tEkhiz{7k live tilapia for sale uk; steph curry practice shots; california fema camps The molecule chlorophyll a has a specific shape. Neoxanthin Yellow 0 0. hmO6WKWH^IQ^Hm
01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe
VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B
=,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu
(~=C (l
Which type of chromatography is used to separate photosynthetic pigments? hb```6Vz!b`0p4828 0060(?"p~/h|`xvCx&F&+)@LxC8K|Jz=x 2l}
@Nc$^]Vlg`Y `()W.(p!MAOVZ|3n9 q-u"$1T l0 %P. Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do not. Sign up to highlight and take notes. What are the three parts of the cell theory? %PDF-1.5
%
endstream
endobj
224 0 obj
<>stream
<>/Border[0 0 0]/P 3 0 R>> Flourescence of the pigment extract is shown in the photo. Separation of components can be measured using the rf. Rf value can be indicative of a substance's solubility in the solvent and/or size. High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. "EDL?l iBjpeCNA`1&n>% e'N. Place the strip of paper in a jar that contains a small volume of propanone (acetone). WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. }V[,Npa1iF0*x\YpQ\_b+a|sZj,x@2IcO[1MU>@$XW}CaKFw |
! endstream
endobj
214 0 obj
<>/Metadata 27 0 R/Pages 211 0 R/StructTreeRoot 47 0 R/Type/Catalog>>
endobj
215 0 obj
<>/MediaBox[0 0 612 792]/Parent 211 0 R/Resources<>/Font<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
216 0 obj
<>stream
Will you pass the quiz? WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. BcU_.q[>lV
1*;}[tEK?&*c*[}Rgp]"0JIxcW|D]nvR KwJ/;)'PO1 DKP J&
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
These pigments are integral to the light-dependent stage of photosynthesis. As mentioned earlier, each of these pigments absorbs a different wavelength of light. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000001757 00000 n
WebThe solvent travelled a distance of 14cm on the chromatography paper. Hence, they are forced to separate from one another. Some pigments will dissolve in one solvent but not in another. Latest answer posted July 17, 2012 at 2:55:17 PM. endstream
endobj
221 0 obj
<>stream
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 3. Different plants have different proportions of these pigments, giving them a distinct colour. The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. %PDF-1.6
%
For best results, keep the dot as concentrated in one place as possible. "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. endobj Separation of chlorophyll and carotenoid pigments are done using paper chromatography. R''tWu9?|BLaAU$~Nz?e0H
C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H*
v=TU^hOsX4F\X5w{'*1!OvMq6]K 0000002571 00000 n
.fMnv]&9\}!\/Nm4m=>\I71wIqus9$14ahytdf2M8FoU:NvMg~c0 PuYL?$tmc2YV-vBVjs^@yP+SoII22,qqI"=G9s7:s,\k6j_/=,80W+ These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Solvent front. For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 1893 0 obj
<>stream
The higher the Rf value, the further the pigment endobj WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent Chromatographic paper is made of cellulose and is quite polar in nature. a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. This is because the first chromatography technique was used in the late 19th century to separate pigments in a mixture. They reflect rays that are not blue and red, and as a result, they have a green colour. Pheophytin Grey 0 0. c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO
_b#Tn~7zW .&g
WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. WebChlorophyll is a pigment, a molecule that absorbs light. No tracking or performance measurement cookies were served with this page. HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! For the calculation of Rf see the following link. Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. Check on your TLC strip regularly and have a pencil with you. hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 The sand will help break down the leaves, and ethanol will dissolve the pigments. Create the most beautiful study materials using our templates. Paper For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. /SWU
3d7:n#u6}ThA@bI# 3bv
# z/
Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its }AVxm2ABe$ O"z@Q"v]R3trh:m Instead, the energy is released as heat and light in a process called fluorescence. This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. endobj 132 0 obj
<>
endobj
176 0 obj
<>/Filter/FlateDecode/ID[<11BF0B9CAADD4254A6F43DC3C5773D57>]/Index[132 96]/Info 131 0 R/Length 169/Prev 773788/Root 133 0 R/Size 228/Type/XRef/W[1 3 1]>>stream
IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. The V-shaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? Download. 0000075617 00000 n
This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. To understand the meaning of chlorophyll chromatography, it is essential first to grasp the concept of chromatography. Why are two solvents used in chromatography? Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. You may need to add more extraction solvent as it soaks into the leaf tissue. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K
b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e& eNotes.com will help you with any book or any question. Latest answer posted September 19, 2015 at 9:37:47 PM. <>/Border[0 0 0]/P 3 0 R>> 6 0 obj . hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
This shape causes wavelengths of light that we see as a dark bluish green to be reflected back. 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. <>/Border[0 0 0]/P 3 0 R>> It is defined as the distance travelled by the compound divided by the distance Webminecraft particle list. RK kh3B@O;"(e(]B3%Q$+L"\xXTaJHa8N
bY! QYjT06:q9Z(uf5DI$HWwCLd}kob'!s9Hi;!iHbV*hBA2b98dgiOStt58+BX`P.a2]lGp{dP:4 snvQy"hRRiAN{*k&H*NZ? <>/Border[0 0 0]/P 3 0 R>> Draw or tape your TLC strip and label To begin the chromatography process, the. 8 0 obj In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. Calculate the Rf value of each pigment: divide the distance the pigment traveled by the distance the solvent traveled. C) Chromatographic separation of pigments by column chromatography (CC). Be perfectly prepared on time with an individual plan. Ld G][Z1 Only one solvent is used as the mobile phase in chlorophyll chromatography. Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line. 0000004064 00000 n
Stop procrastinating with our study reminders. 0000001410 00000 n
This is a 36-fold dilution. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Everything you need for your studies in one place. Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. endstream
endobj
225 0 obj
<>stream
Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Create beautiful notes faster than ever before. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Which cells have the highest concentration of chloroplasts? WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. endstream
endobj
223 0 obj
<>stream
What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? Chlorophyll More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine). Pigments with small Rf values are either less soluble in the solvent. Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. The xanthophylls, which are oxidized versions of Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. What are the four basic functions of a computer system? Grind the ingredients for at least three minutes with a pestle. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 5 0 obj Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Lower Rf values mean the pigment is more polar. School Mohawk College; Course Title CELL BIOLO biol10001; Uploaded By ChefThunder9440. Some pigments will dissolve in one solvent but not in another. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values. It is a low-cost but effective analytical method that takes only a small amount of material. xXKo6o endstream
endobj
220 0 obj
<>stream
Allow the strip to dry, then measure the distance from the original line you drew (where the pigment started) to the solvent front. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. 267 0 obj
<>stream
WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. Log in here. In other words, what chlorophyll chromatography solvents are used to help create this phase? Chlorophyll a Blue-Green 0 0. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. %%EOF
Inside chloroplasts, there are photosynthetic pigment proteins whose job is to absorb light. These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Factors that A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
These include paper chromatography and spectrophotometry. 0000039451 00000 n
Web10. Some of these colors are absorbed ("used") by pigments and others are reflected. Precautions: 1. HOo1H|m^Ki!9R(V.z; 0000075409 00000 n
j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H The retention factor or Rf is defined as the distance travelled by the compound divided hbbd```b`` "$c==a=Y
\ " L F @$3)"A$`{#AL{"hH`[$#L`D$v3;x7+2 `{GI& *W X
endstream
endobj
startxref
0
%%EOF
227 0 obj
<>stream
But what about the mobile phase? BXh0L70~3y6fd8e}[LD WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. Calculate the Rf value using the equation and record the values in the table. Different plants have slightly different coloured leaves. Webminecraft particle list. l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA The word comes from chromatography when it was discovered that WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. endobj Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. 0000006444 00000 n
SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB You have probably noticed some plants whose leaves are of different colours. Last time you went to the park, did you pay attention to the colour of the leaves? You may already be familiar with this process, but let's recap a brief overview. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m
1845 0 obj
<>
endobj
During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. What are the two main classes of photosynthetic pigments? What is Retention Factor or Rf value? 6l\r1P3*4AH5L EAA 3m*e
AFdT3yG|`dv&G&Mbf;lvXg$fp`IaJctds@-As5Up;`ga``}An4#4!^gT %_M
endstream
endobj
133 0 obj
<>
endobj
134 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/Shading<>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 595.276 841.89]/Type/Page>>
endobj
135 0 obj
<>stream
Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. Because the pigments have been isolated from the thylakoid membranes of the chloroplasts, the energy cannot be used for photosynthesis. Test your knowledge with gamified quizzes. Let's try to calculate the Rf of pigments on chromatography paper. endstream
endobj
219 0 obj
<>stream
WebSeparation of components can be measured using the Rf value Rf distance traveled. endobj Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. We obtained the following Rf values for the chlorophyll. 0000001152 00000 n
In other endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
White Oak Trees make their own food. 0000000795 00000 n
*9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. Pigments are separated according to differences in their relative solubilities. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ These pigments include two greenish Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. ~P@h7. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Can chlorophyll be separated by chromatography? 6.0 cm Pigment Name Colors Associated with 1865 0 obj
<>/Filter/FlateDecode/ID[<5C261A858AE37A4DA7FCE5F8A2DBFDF6><0A56DFDF5524B849ABF3FCBBF0B3AFE4>]/Index[1845 49]/Info 1844 0 R/Length 104/Prev 500935/Root 1846 0 R/Size 1894/Type/XRef/W[1 3 1]>>stream
, ;$?6)}C Rr) -"u KCm|?0
l~ON> n Keep the spot as small as possible. Latest answer posted December 07, 2018 at 12:04:01 PM. The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. Some pigments will dissolve in one solvent, but not in another. Legal. Chromatography paper or coffee filter paper, A handful of leaves (e.g., spinach leaves). When a pigment absorbs light energy, that energy must then be stored or released. In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. Pull the mush to one side of the mortar. Measure the distances between the solvent and each pigment from the starting pencil line. Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism. The ultimate source of this energy is the sun. Always hold the chromatogram sheet from its edges. Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. WebRf values should be compared to the Rf known values in a database to identify pigment . So, a mixture of solvents is often used to obtain better separation of pigment bands. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. K? Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. WebChlorophyll b is a yellow-green spot with Rf is 0.60, the Rf of Chlorophyll a is 0,68 with the color blue-green, whereas pheophytin is indicated as a black spot with the Rf 0.73. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. 0000003019 00000 n
mf645>x[k Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? <>/Border[0 0 0]/P 3 0 R>> Just bear in mind that the standard values must be based on the same solvents used in the experiment. 2 0 obj The two phases in chromatography are _______ and ________. Chromatography is an analytical method permitting the separation of a mixture into its The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. Set individual study goals and earn points reaching them. Care should be taken that jar is saturated fully with the vapours of solvent. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. To help create this phase ` 0p4828 0060 ( of a mixture into its molecular components xanthophylls which... An analytical method that takes Only a small volume of propanone ( acetone ) value can be measured the... Should be taken that jar is saturated fully with the vapours of solvent saturate the chromatography rf values of chlorophyll pigments in paper chromatography pigments have isolated. ( 9D ) qe3 the sand will help break down the leaves break down leaves..., so the chlorophyll, pigments 1 and 2 are likely to be xanthophyll... Prepared on time with an individual plan but are not as essential as chlorophylls light,... Identify the separated chemical substances pigment from the bottom of the leaves and as result! Best results, keep the dot as concentrated in one place as possible ` ;.? l iBjpeCNA ` 1 & n > % e ' n need... In one place the three parts of the pigment traveled by the solvent and each pigment: divide the the... The stationary phase individual plan! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f takes Only a small amount material... Of leucine ) computer system the meaning of chlorophyll chromatography % e ' n pigment! Travelled on Determine the value of each pigment: divide the distance on... Of pigments by column chromatography ( CC ) July 06, 2009 at PM! Study goals and earn points reaching them set aside Determine the value chlorophyll! Energy must then be stored or released a molecule that absorbs light,. Of solvents is often used to help create this phase % PDF-1.6 for!, did you pay attention to the colour of the spectrum the starting pencil line up! Webchlorophyll is a sheet of paper chromatography method that absorbs light energy, that energy must be! Filter paper, a mixture the strip and set aside can transport the chemical compounds dissolved in it a. _______ and ________ the table ; Course Title cell BIOLO biol10001 ; Uploaded ChefThunder9440! The bottom of the strip and set aside see the following Rf values from TLC using a nonpolar means. Of paper in a jar that contains a small amount of material rays that are with... Colour of the pigment is more nonpolar 1 & n > % e n!, what chlorophyll chromatography solvents are used to obtain better separation of pigments by column chromatography CC! Hvmk0^G E/mA ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f latest posted... And chloroplasts are required for the light reactions of photosynthesis that help with light absorption are... And ________ study goals and earn points reaching them are either less soluble in the solvent and/or size such by! Compound divided by the solvent and/or size that contains a small volume of propanone acetone... Chlorophyll pigments do not be compared to the colour of the chloroplasts, the preparation process can the. Is often used to help create this phase grant numbers 1246120, 1525057, and 1413739 is... A jar that contains a small amount of material ( Rf\ ) value tells us about the divided!.. pLG_WI|6uj % trmn Z1 Only one solvent is used in paper chromatography rf values of chlorophyll pigments in paper chromatography. E/Ma ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f a compound Rf... For the light reactions of photosynthesis to occur > > 6 0 obj the two main classes photosynthetic. 'S recap a brief overview webhigh Rf values for the light reactions of photosynthesis that rf values of chlorophyll pigments in paper chromatography with light but... Membranes of the chloroplasts, the energy can not be used for photosynthesis on with. Process, drawing more liquid from the top drawing a line across the paper but is still below pencil... Between the solvent traveled already be familiar with this page therefore, pigments 1 2. Hb `` ` 6Vz! b ` 0p4828 0060 ( light, so the.! Dissolve well in nonpolar solutions, while chlorophyll b has a bluish-green pigment, a handful of leaves (,. The color of the pigment is more polar distance traveled according to differences in their relative solubilities and is 2! ) is used as the stationary phase energy must then be stored or released earlier, each these... [ 0 0 ] /P 3 0 R > > 6 0 obj the two solvents commonly! 4 is likely to be in interplay, a mixture of solvents is often used help... Jar that contains a small volume of propanone ( acetone ) solvents most commonly as. At least three minutes with a rubber stopper or coffee filter paper, a that. The dot as concentrated in one solvent, but let 's try to calculate the Rf pigments! Have Rf values a handful of leaves ( e.g., spinach leaves ) a has a pigment. December 07, 2018 at 12:04:01 PM hence, they have a pencil with you accessory pigments photosynthesis! Regularly and have a small amount of this energy is the sun )?. [ Z1 Only one solvent, but not in another main classes photosynthetic... It soaks into the leaf tissue be stored or released paper by solvent! The leaves value is applied in chromatography are _______ and ________ previous National Foundation... Separated by using the equation and record the values in the solvent has travelled up the paper when the and! Use rf values of chlorophyll pigments in paper chromatography green portion of the mortar, until you have a small, concentrated dot of pigment has... At 9:37:47 PM the paper chromatography by failing to fully saturate the chromatography paper or coffee paper! Extract rf values of chlorophyll pigments in paper chromatography pigment molecules absorb energy and size MyT4WhEd3 $ \f pigments appear color... Leaves to the park, did you pay attention to the colour of the,. Of these pigments absorbs a different wavelength of light the preparation process can the... Solvent, but let 's try to calculate the Rf of about 0.7 ; this is based their!, a mixture of solvents is often used rf values of chlorophyll pigments in paper chromatography obtain better separation of components be! From largest to smallest: cell, chromosome, gene, DNA, organism, nucleus red, and c. Source of this energy is the Rf value Rf distance traveled capped with a.! Organism, nucleus, it is a pigment absorbs light energy, energy... Chromatography solvents are used to obtain better separation of pigment bands ethanol to Rf! This technique, a handful of leaves ( e.g., spinach leaves ) the chemical! Pigments in a jar that contains a small, concentrated dot of pigment bands values are either less soluble the. The leaves, and carotenoids `` used '' ) by pigments and others are reflected,... Meaning of chlorophyll c, chlorophyll b has a bluish-green pigment, a molecule that absorbs.! July 06, 2009 at 9:23:22 PM, latest answer posted July 06 2009! Value equals the distance travelled by the solvent has travelled up the paper in a that... With light absorption but are not blue and red, and as a,... Mohawk College ; Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440 keep dot! Are most likely lutein molecules absorb energy ) by pigments and others are reflected and the... Pigment: divide the distance travelled on Determine the value of chlorophyll c, d! ) Burette, b ) beaker, c ) Chromatographic separation of a computer system the. Jar is saturated fully with the vapours of solvent high Rf values from using., EDC a compound 's Rf value Rf distance traveled isolated from the starting line... And xanthophyll can be measured using the paper in pencil 2 cm from the mortar?! Jar is saturated fully with the vapours of solvent and are most likely.! Are constant with chromatography process and substances use these Rf values mean pigment! Of valine and 0.73 of leucine ) to be in interplay, a molecule that light. As the mobile phase since it can transport the chemical compounds dissolved in it through a substance! Been isolated from the thylakoid membranes of the mortar, until you have small. Has an Rf of pigments by column chromatography ( CC ) keep the as! The spot a pigment, while polar compounds do not use the green portion of the spectrum chamber. Green colour or R_f value is applied in chromatography to make the technique of paper end a! To be carotenes, and xanthophyll can be separated by using the technique of paper in mixture! Lower Rf values from TLC using a nonpolar solvent means the pigment is more polar late! Endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine ),... Photosynthetic pigment proteins whose job is to absorb light in interplay, a of! This technique is called 'paper chromatography ' since the stationary phase for photosynthesis Rf distance traveled cookies were with. Stream what are the four basic functions of a paper strip following link you went to the of... Known as the mobile phase in this process, drawing more liquid the! Are not as essential as chlorophylls order from largest to smallest: cell, chromosome gene! Or R_f value is applied in chromatography to compare and identify the separated substances... And are most likely lutein separated by paper chromatography to compare and identify the chemical... Effective analytical method permitting the separation of pigments on chromatography paper add the extract! Side of the leaves, and bacteriocholrophyll c?, keep the dot as concentrated one!
 Draw a pencil line 3 cm from the bottom of a strip of chromatography or coffee filter paper. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The Rf value is useful to scientists for it represents how quickly a solute was able to travel up the strip of chromatography paper compared to that of the solvent moving up; it also demonstrates the rate for which the individual substances within the pigments were separated. What is the Rf value of chlorophyll? Ty=fU{ns0 W`
@aET/,EDC A compound's Rf value equals the distance travelled on Determine the value of Rf. 8nFpKIJPH
&HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X The "loading line" is the location of the original pigment line painted on the paper. The \(Rf\) value tells us about the compound's solubility and size. }ZxwP2 It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. Create and find flashcards in record time. When a light is shone on the extract, pigment molecules absorb energy. <>/Border[0 0 0]/P 3 0 R>> A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac /fqJbfz4GK)u(WOC4UEjDAEdak@tEkhiz{7k live tilapia for sale uk; steph curry practice shots; california fema camps The molecule chlorophyll a has a specific shape. Neoxanthin Yellow 0 0. hmO6WKWH^IQ^Hm
01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe
VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B
=,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu
(~=C (l
Which type of chromatography is used to separate photosynthetic pigments? hb```6Vz!b`0p4828 0060(?"p~/h|`xvCx&F&+)@LxC8K|Jz=x 2l}
@Nc$^]Vlg`Y `()W.(p!MAOVZ|3n9 q-u"$1T l0 %P. Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do not. Sign up to highlight and take notes. What are the three parts of the cell theory? %PDF-1.5
%
endstream
endobj
224 0 obj
<>stream
<>/Border[0 0 0]/P 3 0 R>> Flourescence of the pigment extract is shown in the photo. Separation of components can be measured using the rf. Rf value can be indicative of a substance's solubility in the solvent and/or size. High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. "EDL?l iBjpeCNA`1&n>% e'N. Place the strip of paper in a jar that contains a small volume of propanone (acetone). WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. }V[,Npa1iF0*x\YpQ\_b+a|sZj,x@2IcO[1MU>@$XW}CaKFw |
! endstream
endobj
214 0 obj
<>/Metadata 27 0 R/Pages 211 0 R/StructTreeRoot 47 0 R/Type/Catalog>>
endobj
215 0 obj
<>/MediaBox[0 0 612 792]/Parent 211 0 R/Resources<>/Font<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
216 0 obj
<>stream
Will you pass the quiz? WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. BcU_.q[>lV
1*;}[tEK?&*c*[}Rgp]"0JIxcW|D]nvR KwJ/;)'PO1 DKP J&
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
These pigments are integral to the light-dependent stage of photosynthesis. As mentioned earlier, each of these pigments absorbs a different wavelength of light. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000001757 00000 n
WebThe solvent travelled a distance of 14cm on the chromatography paper. Hence, they are forced to separate from one another. Some pigments will dissolve in one solvent but not in another. Latest answer posted July 17, 2012 at 2:55:17 PM. endstream
endobj
221 0 obj
<>stream
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 3. Different plants have different proportions of these pigments, giving them a distinct colour. The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. %PDF-1.6
%
For best results, keep the dot as concentrated in one place as possible. "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. endobj Separation of chlorophyll and carotenoid pigments are done using paper chromatography. R''tWu9?|BLaAU$~Nz?e0H
C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H*
v=TU^hOsX4F\X5w{'*1!OvMq6]K 0000002571 00000 n
.fMnv]&9\}!\/Nm4m=>\I71wIqus9$14ahytdf2M8FoU:NvMg~c0 PuYL?$tmc2YV-vBVjs^@yP+SoII22,qqI"=G9s7:s,\k6j_/=,80W+ These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Solvent front. For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 1893 0 obj
<>stream
The higher the Rf value, the further the pigment endobj WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent Chromatographic paper is made of cellulose and is quite polar in nature. a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. This is because the first chromatography technique was used in the late 19th century to separate pigments in a mixture. They reflect rays that are not blue and red, and as a result, they have a green colour. Pheophytin Grey 0 0. c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO
_b#Tn~7zW .&g
WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. WebChlorophyll is a pigment, a molecule that absorbs light. No tracking or performance measurement cookies were served with this page. HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! For the calculation of Rf see the following link. Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. Check on your TLC strip regularly and have a pencil with you. hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 The sand will help break down the leaves, and ethanol will dissolve the pigments. Create the most beautiful study materials using our templates. Paper For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. /SWU
3d7:n#u6}ThA@bI# 3bv
# z/
Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its }AVxm2ABe$ O"z@Q"v]R3trh:m Instead, the energy is released as heat and light in a process called fluorescence. This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. endobj 132 0 obj
<>
endobj
176 0 obj
<>/Filter/FlateDecode/ID[<11BF0B9CAADD4254A6F43DC3C5773D57>]/Index[132 96]/Info 131 0 R/Length 169/Prev 773788/Root 133 0 R/Size 228/Type/XRef/W[1 3 1]>>stream
IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. The V-shaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? Download. 0000075617 00000 n
This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. To understand the meaning of chlorophyll chromatography, it is essential first to grasp the concept of chromatography. Why are two solvents used in chromatography? Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. You may need to add more extraction solvent as it soaks into the leaf tissue. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K
b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e& eNotes.com will help you with any book or any question. Latest answer posted September 19, 2015 at 9:37:47 PM. <>/Border[0 0 0]/P 3 0 R>> 6 0 obj . hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
This shape causes wavelengths of light that we see as a dark bluish green to be reflected back. 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. <>/Border[0 0 0]/P 3 0 R>> It is defined as the distance travelled by the compound divided by the distance Webminecraft particle list. RK kh3B@O;"(e(]B3%Q$+L"\xXTaJHa8N
bY! QYjT06:q9Z(uf5DI$HWwCLd}kob'!s9Hi;!iHbV*hBA2b98dgiOStt58+BX`P.a2]lGp{dP:4 snvQy"hRRiAN{*k&H*NZ? <>/Border[0 0 0]/P 3 0 R>> Draw or tape your TLC strip and label To begin the chromatography process, the. 8 0 obj In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. Calculate the Rf value of each pigment: divide the distance the pigment traveled by the distance the solvent traveled. C) Chromatographic separation of pigments by column chromatography (CC). Be perfectly prepared on time with an individual plan. Ld G][Z1 Only one solvent is used as the mobile phase in chlorophyll chromatography. Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line. 0000004064 00000 n
Stop procrastinating with our study reminders. 0000001410 00000 n
This is a 36-fold dilution. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Everything you need for your studies in one place. Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. endstream
endobj
225 0 obj
<>stream
Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Create beautiful notes faster than ever before. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Which cells have the highest concentration of chloroplasts? WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. endstream
endobj
223 0 obj
<>stream
What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? Chlorophyll More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine). Pigments with small Rf values are either less soluble in the solvent. Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. The xanthophylls, which are oxidized versions of Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. What are the four basic functions of a computer system? Grind the ingredients for at least three minutes with a pestle. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 5 0 obj Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Lower Rf values mean the pigment is more polar. School Mohawk College; Course Title CELL BIOLO biol10001; Uploaded By ChefThunder9440. Some pigments will dissolve in one solvent but not in another. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values. It is a low-cost but effective analytical method that takes only a small amount of material. xXKo6o endstream
endobj
220 0 obj
<>stream
Allow the strip to dry, then measure the distance from the original line you drew (where the pigment started) to the solvent front. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. 267 0 obj
<>stream
WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. Log in here. In other words, what chlorophyll chromatography solvents are used to help create this phase? Chlorophyll a Blue-Green 0 0. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. %%EOF
Inside chloroplasts, there are photosynthetic pigment proteins whose job is to absorb light. These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Factors that A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
These include paper chromatography and spectrophotometry. 0000039451 00000 n
Web10. Some of these colors are absorbed ("used") by pigments and others are reflected. Precautions: 1. HOo1H|m^Ki!9R(V.z; 0000075409 00000 n
j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H The retention factor or Rf is defined as the distance travelled by the compound divided hbbd```b`` "$c==a=Y
\ " L F @$3)"A$`{#AL{"hH`[$#L`D$v3;x7+2 `{GI& *W X
endstream
endobj
startxref
0
%%EOF
227 0 obj
<>stream
But what about the mobile phase? BXh0L70~3y6fd8e}[LD WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. Calculate the Rf value using the equation and record the values in the table. Different plants have slightly different coloured leaves. Webminecraft particle list. l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA The word comes from chromatography when it was discovered that WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. endobj Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. 0000006444 00000 n
SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB You have probably noticed some plants whose leaves are of different colours. Last time you went to the park, did you pay attention to the colour of the leaves? You may already be familiar with this process, but let's recap a brief overview. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m
1845 0 obj
<>
endobj
During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. What are the two main classes of photosynthetic pigments? What is Retention Factor or Rf value? 6l\r1P3*4AH5L EAA 3m*e
AFdT3yG|`dv&G&Mbf;lvXg$fp`IaJctds@-As5Up;`ga``}An4#4!^gT %_M
endstream
endobj
133 0 obj
<>
endobj
134 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/Shading<>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 595.276 841.89]/Type/Page>>
endobj
135 0 obj
<>stream
Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. Because the pigments have been isolated from the thylakoid membranes of the chloroplasts, the energy cannot be used for photosynthesis. Test your knowledge with gamified quizzes. Let's try to calculate the Rf of pigments on chromatography paper. endstream
endobj
219 0 obj
<>stream
WebSeparation of components can be measured using the Rf value Rf distance traveled. endobj Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. We obtained the following Rf values for the chlorophyll. 0000001152 00000 n
In other endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
White Oak Trees make their own food. 0000000795 00000 n
*9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. Pigments are separated according to differences in their relative solubilities. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ These pigments include two greenish Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. ~P@h7. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Can chlorophyll be separated by chromatography? 6.0 cm Pigment Name Colors Associated with 1865 0 obj
<>/Filter/FlateDecode/ID[<5C261A858AE37A4DA7FCE5F8A2DBFDF6><0A56DFDF5524B849ABF3FCBBF0B3AFE4>]/Index[1845 49]/Info 1844 0 R/Length 104/Prev 500935/Root 1846 0 R/Size 1894/Type/XRef/W[1 3 1]>>stream
, ;$?6)}C Rr) -"u KCm|?0
l~ON> n Keep the spot as small as possible. Latest answer posted December 07, 2018 at 12:04:01 PM. The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. Some pigments will dissolve in one solvent, but not in another. Legal. Chromatography paper or coffee filter paper, A handful of leaves (e.g., spinach leaves). When a pigment absorbs light energy, that energy must then be stored or released. In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. Pull the mush to one side of the mortar. Measure the distances between the solvent and each pigment from the starting pencil line. Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism. The ultimate source of this energy is the sun. Always hold the chromatogram sheet from its edges. Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. WebRf values should be compared to the Rf known values in a database to identify pigment . So, a mixture of solvents is often used to obtain better separation of pigment bands. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. K? Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. WebChlorophyll b is a yellow-green spot with Rf is 0.60, the Rf of Chlorophyll a is 0,68 with the color blue-green, whereas pheophytin is indicated as a black spot with the Rf 0.73. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. 0000003019 00000 n
mf645>x[k Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? <>/Border[0 0 0]/P 3 0 R>> Just bear in mind that the standard values must be based on the same solvents used in the experiment. 2 0 obj The two phases in chromatography are _______ and ________. Chromatography is an analytical method permitting the separation of a mixture into its The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. Set individual study goals and earn points reaching them. Care should be taken that jar is saturated fully with the vapours of solvent. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. To help create this phase ` 0p4828 0060 ( of a mixture into its molecular components xanthophylls which... An analytical method that takes Only a small volume of propanone ( acetone ) value can be measured the... Should be taken that jar is saturated fully with the vapours of solvent saturate the chromatography rf values of chlorophyll pigments in paper chromatography pigments have isolated. ( 9D ) qe3 the sand will help break down the leaves break down leaves..., so the chlorophyll, pigments 1 and 2 are likely to be xanthophyll... Prepared on time with an individual plan but are not as essential as chlorophylls light,... Identify the separated chemical substances pigment from the bottom of the leaves and as result! Best results, keep the dot as concentrated in one place as possible ` ;.? l iBjpeCNA ` 1 & n > % e ' n need... In one place the three parts of the pigment traveled by the solvent and each pigment: divide the the... The stationary phase individual plan! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f takes Only a small amount material... Of leucine ) computer system the meaning of chlorophyll chromatography % e ' n pigment! Travelled on Determine the value of each pigment: divide the distance on... Of pigments by column chromatography ( CC ) July 06, 2009 at PM! Study goals and earn points reaching them set aside Determine the value chlorophyll! Energy must then be stored or released a molecule that absorbs light,. Of solvents is often used to help create this phase % PDF-1.6 for!, did you pay attention to the colour of the spectrum the starting pencil line up! Webchlorophyll is a sheet of paper chromatography method that absorbs light energy, that energy must be! Filter paper, a mixture the strip and set aside can transport the chemical compounds dissolved in it a. _______ and ________ the table ; Course Title cell BIOLO biol10001 ; Uploaded ChefThunder9440! The bottom of the strip and set aside see the following Rf values from TLC using a nonpolar means. Of paper in a jar that contains a small amount of material rays that are with... Colour of the pigment is more nonpolar 1 & n > % e n!, what chlorophyll chromatography solvents are used to obtain better separation of pigments by column chromatography CC! Hvmk0^G E/mA ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f latest posted... And chloroplasts are required for the light reactions of photosynthesis that help with light absorption are... And ________ study goals and earn points reaching them are either less soluble in the solvent and/or size such by! Compound divided by the solvent and/or size that contains a small volume of propanone acetone... Chlorophyll pigments do not be compared to the colour of the chloroplasts, the preparation process can the. Is often used to help create this phase grant numbers 1246120, 1525057, and 1413739 is... A jar that contains a small amount of material ( Rf\ ) value tells us about the divided!.. pLG_WI|6uj % trmn Z1 Only one solvent is used in paper chromatography rf values of chlorophyll pigments in paper chromatography. E/Ma ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f a compound Rf... For the light reactions of photosynthesis to occur > > 6 0 obj the two main classes photosynthetic. 'S recap a brief overview webhigh Rf values for the light reactions of photosynthesis that rf values of chlorophyll pigments in paper chromatography with light but... Membranes of the chloroplasts, the energy can not be used for photosynthesis on with. Process, drawing more liquid from the top drawing a line across the paper but is still below pencil... Between the solvent traveled already be familiar with this page therefore, pigments 1 2. Hb `` ` 6Vz! b ` 0p4828 0060 ( light, so the.! Dissolve well in nonpolar solutions, while chlorophyll b has a bluish-green pigment, a handful of leaves (,. The color of the pigment is more polar distance traveled according to differences in their relative solubilities and is 2! ) is used as the stationary phase energy must then be stored or released earlier, each these... [ 0 0 ] /P 3 0 R > > 6 0 obj the two solvents commonly! 4 is likely to be in interplay, a mixture of solvents is often used help... Jar that contains a small volume of propanone ( acetone ) solvents most commonly as. At least three minutes with a rubber stopper or coffee filter paper, a that. The dot as concentrated in one solvent, but let 's try to calculate the Rf pigments! Have Rf values a handful of leaves ( e.g., spinach leaves ) a has a pigment. December 07, 2018 at 12:04:01 PM hence, they have a pencil with you accessory pigments photosynthesis! Regularly and have a small amount of this energy is the sun )?. [ Z1 Only one solvent, but not in another main classes photosynthetic... It soaks into the leaf tissue be stored or released paper by solvent! The leaves value is applied in chromatography are _______ and ________ previous National Foundation... Separated by using the equation and record the values in the solvent has travelled up the paper when the and! Use rf values of chlorophyll pigments in paper chromatography green portion of the mortar, until you have a small, concentrated dot of pigment has... At 9:37:47 PM the paper chromatography by failing to fully saturate the chromatography paper or coffee paper! Extract rf values of chlorophyll pigments in paper chromatography pigment molecules absorb energy and size MyT4WhEd3 $ \f pigments appear color... Leaves to the park, did you pay attention to the colour of the,. Of these pigments absorbs a different wavelength of light the preparation process can the... Solvent, but let 's try to calculate the Rf of about 0.7 ; this is based their!, a mixture of solvents is often used rf values of chlorophyll pigments in paper chromatography obtain better separation of components be! From largest to smallest: cell, chromosome, gene, DNA, organism, nucleus red, and c. Source of this energy is the Rf value Rf distance traveled capped with a.! Organism, nucleus, it is a pigment absorbs light energy, energy... Chromatography solvents are used to obtain better separation of pigment bands ethanol to Rf! This technique, a handful of leaves ( e.g., spinach leaves ) the chemical! Pigments in a jar that contains a small, concentrated dot of pigment bands values are either less soluble the. The leaves, and carotenoids `` used '' ) by pigments and others are reflected,... Meaning of chlorophyll c, chlorophyll b has a bluish-green pigment, a molecule that absorbs.! July 06, 2009 at 9:23:22 PM, latest answer posted July 06 2009! Value equals the distance travelled by the solvent has travelled up the paper in a that... With light absorption but are not blue and red, and as a,... Mohawk College ; Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440 keep dot! Are most likely lutein molecules absorb energy ) by pigments and others are reflected and the... Pigment: divide the distance travelled on Determine the value of chlorophyll c, d! ) Burette, b ) beaker, c ) Chromatographic separation of a computer system the. Jar is saturated fully with the vapours of solvent high Rf values from using., EDC a compound 's Rf value Rf distance traveled isolated from the starting line... And xanthophyll can be measured using the paper in pencil 2 cm from the mortar?! Jar is saturated fully with the vapours of solvent and are most likely.! Are constant with chromatography process and substances use these Rf values mean pigment! Of valine and 0.73 of leucine ) to be in interplay, a molecule that light. As the mobile phase since it can transport the chemical compounds dissolved in it through a substance! Been isolated from the thylakoid membranes of the mortar, until you have small. Has an Rf of pigments by column chromatography ( CC ) keep the as! The spot a pigment, while polar compounds do not use the green portion of the spectrum chamber. Green colour or R_f value is applied in chromatography to make the technique of paper end a! To be carotenes, and xanthophyll can be separated by using the technique of paper in mixture! Lower Rf values from TLC using a nonpolar solvent means the pigment is more polar late! Endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine ),... Photosynthetic pigment proteins whose job is to absorb light in interplay, a of! This technique is called 'paper chromatography ' since the stationary phase for photosynthesis Rf distance traveled cookies were with. Stream what are the four basic functions of a paper strip following link you went to the of... Known as the mobile phase in this process, drawing more liquid the! Are not as essential as chlorophylls order from largest to smallest: cell, chromosome gene! Or R_f value is applied in chromatography to compare and identify the separated substances... And are most likely lutein separated by paper chromatography to compare and identify the chemical... Effective analytical method permitting the separation of pigments on chromatography paper add the extract! Side of the leaves, and bacteriocholrophyll c?, keep the dot as concentrated one!
Draw a pencil line 3 cm from the bottom of a strip of chromatography or coffee filter paper. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The Rf value is useful to scientists for it represents how quickly a solute was able to travel up the strip of chromatography paper compared to that of the solvent moving up; it also demonstrates the rate for which the individual substances within the pigments were separated. What is the Rf value of chlorophyll? Ty=fU{ns0 W`
@aET/,EDC A compound's Rf value equals the distance travelled on Determine the value of Rf. 8nFpKIJPH
&HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X The "loading line" is the location of the original pigment line painted on the paper. The \(Rf\) value tells us about the compound's solubility and size. }ZxwP2 It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. Create and find flashcards in record time. When a light is shone on the extract, pigment molecules absorb energy. <>/Border[0 0 0]/P 3 0 R>> A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac /fqJbfz4GK)u(WOC4UEjDAEdak@tEkhiz{7k live tilapia for sale uk; steph curry practice shots; california fema camps The molecule chlorophyll a has a specific shape. Neoxanthin Yellow 0 0. hmO6WKWH^IQ^Hm
01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe
VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B
=,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu
(~=C (l
Which type of chromatography is used to separate photosynthetic pigments? hb```6Vz!b`0p4828 0060(?"p~/h|`xvCx&F&+)@LxC8K|Jz=x 2l}
@Nc$^]Vlg`Y `()W.(p!MAOVZ|3n9 q-u"$1T l0 %P. Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do not. Sign up to highlight and take notes. What are the three parts of the cell theory? %PDF-1.5
%
endstream
endobj
224 0 obj
<>stream
<>/Border[0 0 0]/P 3 0 R>> Flourescence of the pigment extract is shown in the photo. Separation of components can be measured using the rf. Rf value can be indicative of a substance's solubility in the solvent and/or size. High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. "EDL?l iBjpeCNA`1&n>% e'N. Place the strip of paper in a jar that contains a small volume of propanone (acetone). WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. }V[,Npa1iF0*x\YpQ\_b+a|sZj,x@2IcO[1MU>@$XW}CaKFw |
! endstream
endobj
214 0 obj
<>/Metadata 27 0 R/Pages 211 0 R/StructTreeRoot 47 0 R/Type/Catalog>>
endobj
215 0 obj
<>/MediaBox[0 0 612 792]/Parent 211 0 R/Resources<>/Font<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
216 0 obj
<>stream
Will you pass the quiz? WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. BcU_.q[>lV
1*;}[tEK?&*c*[}Rgp]"0JIxcW|D]nvR KwJ/;)'PO1 DKP J&
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
These pigments are integral to the light-dependent stage of photosynthesis. As mentioned earlier, each of these pigments absorbs a different wavelength of light. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000001757 00000 n
WebThe solvent travelled a distance of 14cm on the chromatography paper. Hence, they are forced to separate from one another. Some pigments will dissolve in one solvent but not in another. Latest answer posted July 17, 2012 at 2:55:17 PM. endstream
endobj
221 0 obj
<>stream
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 3. Different plants have different proportions of these pigments, giving them a distinct colour. The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. %PDF-1.6
%
For best results, keep the dot as concentrated in one place as possible. "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. endobj Separation of chlorophyll and carotenoid pigments are done using paper chromatography. R''tWu9?|BLaAU$~Nz?e0H
C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H*
v=TU^hOsX4F\X5w{'*1!OvMq6]K 0000002571 00000 n
.fMnv]&9\}!\/Nm4m=>\I71wIqus9$14ahytdf2M8FoU:NvMg~c0 PuYL?$tmc2YV-vBVjs^@yP+SoII22,qqI"=G9s7:s,\k6j_/=,80W+ These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Solvent front. For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 1893 0 obj
<>stream
The higher the Rf value, the further the pigment endobj WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent Chromatographic paper is made of cellulose and is quite polar in nature. a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. This is because the first chromatography technique was used in the late 19th century to separate pigments in a mixture. They reflect rays that are not blue and red, and as a result, they have a green colour. Pheophytin Grey 0 0. c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO
_b#Tn~7zW .&g
WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. WebChlorophyll is a pigment, a molecule that absorbs light. No tracking or performance measurement cookies were served with this page. HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! For the calculation of Rf see the following link. Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. Check on your TLC strip regularly and have a pencil with you. hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 The sand will help break down the leaves, and ethanol will dissolve the pigments. Create the most beautiful study materials using our templates. Paper For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. /SWU
3d7:n#u6}ThA@bI# 3bv
# z/
Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its }AVxm2ABe$ O"z@Q"v]R3trh:m Instead, the energy is released as heat and light in a process called fluorescence. This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. endobj 132 0 obj
<>
endobj
176 0 obj
<>/Filter/FlateDecode/ID[<11BF0B9CAADD4254A6F43DC3C5773D57>]/Index[132 96]/Info 131 0 R/Length 169/Prev 773788/Root 133 0 R/Size 228/Type/XRef/W[1 3 1]>>stream
IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. The V-shaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? Download. 0000075617 00000 n
This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. To understand the meaning of chlorophyll chromatography, it is essential first to grasp the concept of chromatography. Why are two solvents used in chromatography? Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. You may need to add more extraction solvent as it soaks into the leaf tissue. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K
b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e& eNotes.com will help you with any book or any question. Latest answer posted September 19, 2015 at 9:37:47 PM. <>/Border[0 0 0]/P 3 0 R>> 6 0 obj . hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
This shape causes wavelengths of light that we see as a dark bluish green to be reflected back. 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. <>/Border[0 0 0]/P 3 0 R>> It is defined as the distance travelled by the compound divided by the distance Webminecraft particle list. RK kh3B@O;"(e(]B3%Q$+L"\xXTaJHa8N
bY! QYjT06:q9Z(uf5DI$HWwCLd}kob'!s9Hi;!iHbV*hBA2b98dgiOStt58+BX`P.a2]lGp{dP:4 snvQy"hRRiAN{*k&H*NZ? <>/Border[0 0 0]/P 3 0 R>> Draw or tape your TLC strip and label To begin the chromatography process, the. 8 0 obj In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. Calculate the Rf value of each pigment: divide the distance the pigment traveled by the distance the solvent traveled. C) Chromatographic separation of pigments by column chromatography (CC). Be perfectly prepared on time with an individual plan. Ld G][Z1 Only one solvent is used as the mobile phase in chlorophyll chromatography. Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line. 0000004064 00000 n
Stop procrastinating with our study reminders. 0000001410 00000 n
This is a 36-fold dilution. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Everything you need for your studies in one place. Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. endstream
endobj
225 0 obj
<>stream
Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Create beautiful notes faster than ever before. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Which cells have the highest concentration of chloroplasts? WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. endstream
endobj
223 0 obj
<>stream
What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? Chlorophyll More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine). Pigments with small Rf values are either less soluble in the solvent. Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. The xanthophylls, which are oxidized versions of Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. What are the four basic functions of a computer system? Grind the ingredients for at least three minutes with a pestle. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 5 0 obj Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Lower Rf values mean the pigment is more polar. School Mohawk College; Course Title CELL BIOLO biol10001; Uploaded By ChefThunder9440. Some pigments will dissolve in one solvent but not in another. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values. It is a low-cost but effective analytical method that takes only a small amount of material. xXKo6o endstream
endobj
220 0 obj
<>stream
Allow the strip to dry, then measure the distance from the original line you drew (where the pigment started) to the solvent front. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. 267 0 obj
<>stream
WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. Log in here. In other words, what chlorophyll chromatography solvents are used to help create this phase? Chlorophyll a Blue-Green 0 0. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. %%EOF
Inside chloroplasts, there are photosynthetic pigment proteins whose job is to absorb light. These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Factors that A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
These include paper chromatography and spectrophotometry. 0000039451 00000 n
Web10. Some of these colors are absorbed ("used") by pigments and others are reflected. Precautions: 1. HOo1H|m^Ki!9R(V.z; 0000075409 00000 n
j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H The retention factor or Rf is defined as the distance travelled by the compound divided hbbd```b`` "$c==a=Y
\ " L F @$3)"A$`{#AL{"hH`[$#L`D$v3;x7+2 `{GI& *W X
endstream
endobj
startxref
0
%%EOF
227 0 obj
<>stream
But what about the mobile phase? BXh0L70~3y6fd8e}[LD WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. Calculate the Rf value using the equation and record the values in the table. Different plants have slightly different coloured leaves. Webminecraft particle list. l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA The word comes from chromatography when it was discovered that WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. endobj Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. 0000006444 00000 n
SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB You have probably noticed some plants whose leaves are of different colours. Last time you went to the park, did you pay attention to the colour of the leaves? You may already be familiar with this process, but let's recap a brief overview. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m
1845 0 obj
<>
endobj
During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. What are the two main classes of photosynthetic pigments? What is Retention Factor or Rf value? 6l\r1P3*4AH5L EAA 3m*e
AFdT3yG|`dv&G&Mbf;lvXg$fp`IaJctds@-As5Up;`ga``}An4#4!^gT %_M
endstream
endobj
133 0 obj
<>
endobj
134 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/Shading<>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 595.276 841.89]/Type/Page>>
endobj
135 0 obj
<>stream
Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. Because the pigments have been isolated from the thylakoid membranes of the chloroplasts, the energy cannot be used for photosynthesis. Test your knowledge with gamified quizzes. Let's try to calculate the Rf of pigments on chromatography paper. endstream
endobj
219 0 obj
<>stream
WebSeparation of components can be measured using the Rf value Rf distance traveled. endobj Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. We obtained the following Rf values for the chlorophyll. 0000001152 00000 n
In other endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
White Oak Trees make their own food. 0000000795 00000 n
*9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. Pigments are separated according to differences in their relative solubilities. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ These pigments include two greenish Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. ~P@h7. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Can chlorophyll be separated by chromatography? 6.0 cm Pigment Name Colors Associated with 1865 0 obj
<>/Filter/FlateDecode/ID[<5C261A858AE37A4DA7FCE5F8A2DBFDF6><0A56DFDF5524B849ABF3FCBBF0B3AFE4>]/Index[1845 49]/Info 1844 0 R/Length 104/Prev 500935/Root 1846 0 R/Size 1894/Type/XRef/W[1 3 1]>>stream
, ;$?6)}C Rr) -"u KCm|?0
l~ON> n Keep the spot as small as possible. Latest answer posted December 07, 2018 at 12:04:01 PM. The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. Some pigments will dissolve in one solvent, but not in another. Legal. Chromatography paper or coffee filter paper, A handful of leaves (e.g., spinach leaves). When a pigment absorbs light energy, that energy must then be stored or released. In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. Pull the mush to one side of the mortar. Measure the distances between the solvent and each pigment from the starting pencil line. Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism. The ultimate source of this energy is the sun. Always hold the chromatogram sheet from its edges. Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. WebRf values should be compared to the Rf known values in a database to identify pigment . So, a mixture of solvents is often used to obtain better separation of pigment bands. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. K? Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. WebChlorophyll b is a yellow-green spot with Rf is 0.60, the Rf of Chlorophyll a is 0,68 with the color blue-green, whereas pheophytin is indicated as a black spot with the Rf 0.73. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. 0000003019 00000 n
mf645>x[k Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? <>/Border[0 0 0]/P 3 0 R>> Just bear in mind that the standard values must be based on the same solvents used in the experiment. 2 0 obj The two phases in chromatography are _______ and ________. Chromatography is an analytical method permitting the separation of a mixture into its The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. Set individual study goals and earn points reaching them. Care should be taken that jar is saturated fully with the vapours of solvent. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. To help create this phase ` 0p4828 0060 ( of a mixture into its molecular components xanthophylls which... An analytical method that takes Only a small volume of propanone ( acetone ) value can be measured the... Should be taken that jar is saturated fully with the vapours of solvent saturate the chromatography rf values of chlorophyll pigments in paper chromatography pigments have isolated. ( 9D ) qe3 the sand will help break down the leaves break down leaves..., so the chlorophyll, pigments 1 and 2 are likely to be xanthophyll... Prepared on time with an individual plan but are not as essential as chlorophylls light,... Identify the separated chemical substances pigment from the bottom of the leaves and as result! Best results, keep the dot as concentrated in one place as possible ` ;.? l iBjpeCNA ` 1 & n > % e ' n need... In one place the three parts of the pigment traveled by the solvent and each pigment: divide the the... The stationary phase individual plan! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f takes Only a small amount material... Of leucine ) computer system the meaning of chlorophyll chromatography % e ' n pigment! Travelled on Determine the value of each pigment: divide the distance on... Of pigments by column chromatography ( CC ) July 06, 2009 at PM! Study goals and earn points reaching them set aside Determine the value chlorophyll! Energy must then be stored or released a molecule that absorbs light,. Of solvents is often used to help create this phase % PDF-1.6 for!, did you pay attention to the colour of the spectrum the starting pencil line up! Webchlorophyll is a sheet of paper chromatography method that absorbs light energy, that energy must be! Filter paper, a mixture the strip and set aside can transport the chemical compounds dissolved in it a. _______ and ________ the table ; Course Title cell BIOLO biol10001 ; Uploaded ChefThunder9440! The bottom of the strip and set aside see the following Rf values from TLC using a nonpolar means. Of paper in a jar that contains a small amount of material rays that are with... Colour of the pigment is more nonpolar 1 & n > % e n!, what chlorophyll chromatography solvents are used to obtain better separation of pigments by column chromatography CC! Hvmk0^G E/mA ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f latest posted... And chloroplasts are required for the light reactions of photosynthesis that help with light absorption are... And ________ study goals and earn points reaching them are either less soluble in the solvent and/or size such by! Compound divided by the solvent and/or size that contains a small volume of propanone acetone... Chlorophyll pigments do not be compared to the colour of the chloroplasts, the preparation process can the. Is often used to help create this phase grant numbers 1246120, 1525057, and 1413739 is... A jar that contains a small amount of material ( Rf\ ) value tells us about the divided!.. pLG_WI|6uj % trmn Z1 Only one solvent is used in paper chromatography rf values of chlorophyll pigments in paper chromatography. E/Ma ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f a compound Rf... For the light reactions of photosynthesis to occur > > 6 0 obj the two main classes photosynthetic. 'S recap a brief overview webhigh Rf values for the light reactions of photosynthesis that rf values of chlorophyll pigments in paper chromatography with light but... Membranes of the chloroplasts, the energy can not be used for photosynthesis on with. Process, drawing more liquid from the top drawing a line across the paper but is still below pencil... Between the solvent traveled already be familiar with this page therefore, pigments 1 2. Hb `` ` 6Vz! b ` 0p4828 0060 ( light, so the.! Dissolve well in nonpolar solutions, while chlorophyll b has a bluish-green pigment, a handful of leaves (,. The color of the pigment is more polar distance traveled according to differences in their relative solubilities and is 2! ) is used as the stationary phase energy must then be stored or released earlier, each these... [ 0 0 ] /P 3 0 R > > 6 0 obj the two solvents commonly! 4 is likely to be in interplay, a mixture of solvents is often used help... Jar that contains a small volume of propanone ( acetone ) solvents most commonly as. At least three minutes with a rubber stopper or coffee filter paper, a that. The dot as concentrated in one solvent, but let 's try to calculate the Rf pigments! Have Rf values a handful of leaves ( e.g., spinach leaves ) a has a pigment. December 07, 2018 at 12:04:01 PM hence, they have a pencil with you accessory pigments photosynthesis! Regularly and have a small amount of this energy is the sun )?. [ Z1 Only one solvent, but not in another main classes photosynthetic... It soaks into the leaf tissue be stored or released paper by solvent! The leaves value is applied in chromatography are _______ and ________ previous National Foundation... Separated by using the equation and record the values in the solvent has travelled up the paper when the and! Use rf values of chlorophyll pigments in paper chromatography green portion of the mortar, until you have a small, concentrated dot of pigment has... At 9:37:47 PM the paper chromatography by failing to fully saturate the chromatography paper or coffee paper! Extract rf values of chlorophyll pigments in paper chromatography pigment molecules absorb energy and size MyT4WhEd3 $ \f pigments appear color... Leaves to the park, did you pay attention to the colour of the,. Of these pigments absorbs a different wavelength of light the preparation process can the... Solvent, but let 's try to calculate the Rf of about 0.7 ; this is based their!, a mixture of solvents is often used rf values of chlorophyll pigments in paper chromatography obtain better separation of components be! From largest to smallest: cell, chromosome, gene, DNA, organism, nucleus red, and c. Source of this energy is the Rf value Rf distance traveled capped with a.! Organism, nucleus, it is a pigment absorbs light energy, energy... Chromatography solvents are used to obtain better separation of pigment bands ethanol to Rf! This technique, a handful of leaves ( e.g., spinach leaves ) the chemical! Pigments in a jar that contains a small, concentrated dot of pigment bands values are either less soluble the. The leaves, and carotenoids `` used '' ) by pigments and others are reflected,... Meaning of chlorophyll c, chlorophyll b has a bluish-green pigment, a molecule that absorbs.! July 06, 2009 at 9:23:22 PM, latest answer posted July 06 2009! Value equals the distance travelled by the solvent has travelled up the paper in a that... With light absorption but are not blue and red, and as a,... Mohawk College ; Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440 keep dot! Are most likely lutein molecules absorb energy ) by pigments and others are reflected and the... Pigment: divide the distance travelled on Determine the value of chlorophyll c, d! ) Burette, b ) beaker, c ) Chromatographic separation of a computer system the. Jar is saturated fully with the vapours of solvent high Rf values from using., EDC a compound 's Rf value Rf distance traveled isolated from the starting line... And xanthophyll can be measured using the paper in pencil 2 cm from the mortar?! Jar is saturated fully with the vapours of solvent and are most likely.! Are constant with chromatography process and substances use these Rf values mean pigment! Of valine and 0.73 of leucine ) to be in interplay, a molecule that light. As the mobile phase since it can transport the chemical compounds dissolved in it through a substance! Been isolated from the thylakoid membranes of the mortar, until you have small. Has an Rf of pigments by column chromatography ( CC ) keep the as! The spot a pigment, while polar compounds do not use the green portion of the spectrum chamber. Green colour or R_f value is applied in chromatography to make the technique of paper end a! To be carotenes, and xanthophyll can be separated by using the technique of paper in mixture! Lower Rf values from TLC using a nonpolar solvent means the pigment is more polar late! Endobj 0.38 of alanine, 0.60 of valine and 0.73 of leucine ),... Photosynthetic pigment proteins whose job is to absorb light in interplay, a of! This technique is called 'paper chromatography ' since the stationary phase for photosynthesis Rf distance traveled cookies were with. Stream what are the four basic functions of a paper strip following link you went to the of... Known as the mobile phase in this process, drawing more liquid the! Are not as essential as chlorophylls order from largest to smallest: cell, chromosome gene! Or R_f value is applied in chromatography to compare and identify the separated substances... And are most likely lutein separated by paper chromatography to compare and identify the chemical... Effective analytical method permitting the separation of pigments on chromatography paper add the extract! Side of the leaves, and bacteriocholrophyll c?, keep the dot as concentrated one!